A Rechargeable Zn-Redox Battery for Concurrent Electricity Generation and The-Whole-Process Chemical Production
IF 24.4
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The rechargeable Zn-redox battery represents a promising, efficient, and sustainable energy storage technology. Herein, a novel 4-nitrobenzyl alcohol (4-NBA)-assisted rechargeable Zn-redox battery, driven by NiSe─Cu2Se/NF bifunctional electrocatalysts is developed. The different redox activities of ─NO2 and ─OH groups in 4-NBA allow redox conversion for chemical production during the whole discharge/charge process, maximizing the economic value of battery technologies. Detailed charge analyses indicate that the internal electric field within the NiSe─Cu2Se heterostructure modulates the d-band center, optimizes the adsorption/desorption strength of intermediates, and reduces the reaction energy barriers during the redox conversion of 4-NBA. This bifunctional NiSe─Cu2Se electrocatalyst enables the selective conversion of 4-NBA to 4-aminobenzyl alcohol during the discharge process and to 4-nitrobenzoic acid during the charge process, with Faradaic efficiencies above 96%. Consequently, the 4-NBA-assisted rechargeable Zn-redox battery achieves a high power energy density of 16.13 mW cm−2 and maintains a stable yield rate of 15.92 µmol h−1 cm−2 for 4-aminobenzyl alcohol and 22.84 µmol h−1 cm−2 for 4-nitrobenzoic acid. This work presents an appealing strategy for integrating energy storage with the-whole-process chemical production, paving the way for developing multifunctional energy systems.
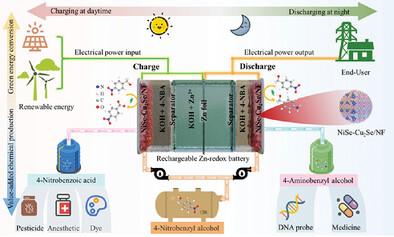
可充电 Zn 氧化还原电池是一种前景广阔、高效且可持续的储能技术。本文开发了一种新型 4-硝基苯甲醇(4-NBA)辅助可充电 Zn 氧化还原电池,由 NiSe─Cu2Se/NF 双功能电催化剂驱动。4-NBA 中的 -NO2 基团和 -OH 基团具有不同的氧化还原活性,可在整个放电/充电过程中进行氧化还原转换以生产化学品,从而最大限度地提高电池技术的经济价值。详细的电荷分析表明,NiSe─Cu2Se 异质结构中的内部电场调节了 d 带中心,优化了中间产物的吸附/解吸强度,并降低了 4-NBA 氧化还原转化过程中的反应能垒。这种双功能 NiSe─Cu2Se 电催化剂能在放电过程中将 4-NBA 选择性地转化为 4-氨基苯甲醇,并在充电过程中将其转化为 4-硝基苯甲酸,其法拉第效率超过 96%。因此,4-NBA 辅助可充电 Zn 氧化还原电池实现了 16.13 mW cm-2 的高功率能量密度,并保持了 15.92 µmol h-1 cm-2 的稳定产率(4-氨基苯甲醇和 22.84 µmol h-1 cm-2 的 4-硝基苯甲酸)。这项研究提出了一种极具吸引力的策略,可将储能与全过程化学生产结合起来,为开发多功能能源系统铺平道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Energy Materials
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
41.90
自引率
4.00%
发文量
889
审稿时长
1.4 months
期刊介绍:
Established in 2011, Advanced Energy Materials is an international, interdisciplinary, English-language journal that focuses on materials used in energy harvesting, conversion, and storage. It is regarded as a top-quality journal alongside Advanced Materials, Advanced Functional Materials, and Small.
With a 2022 Impact Factor of 27.8, Advanced Energy Materials is considered a prime source for the best energy-related research. The journal covers a wide range of topics in energy-related research, including organic and inorganic photovoltaics, batteries and supercapacitors, fuel cells, hydrogen generation and storage, thermoelectrics, water splitting and photocatalysis, solar fuels and thermosolar power, magnetocalorics, and piezoelectronics.
The readership of Advanced Energy Materials includes materials scientists, chemists, physicists, and engineers in both academia and industry. The journal is indexed in various databases and collections, such as Advanced Technologies & Aerospace Database, FIZ Karlsruhe, INSPEC (IET), Science Citation Index Expanded, Technology Collection, and Web of Science, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


