Organic-Coated zeolites for Selective gas Adsorption: Effect of functional group Identity and coating density
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
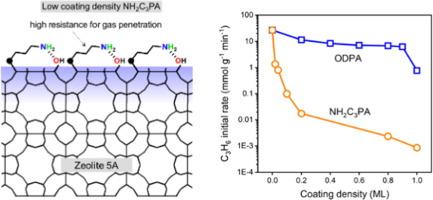
Adsorptive separation of propylene (C3H6) and propane (C3H8) is an alternative to energy-intensive distillation, but improving kinetic selectivity is challenging for molecules with similar sizes. Composite materials consisting of a barrier organic film on the external surface of a zeolite have shown higher selectivity; however, structure–function relationships for these materials are lacking. Here, gas adsorption rates on zeolite 5A were controlled by varying the terminal functional group (amine or carboxylic acid) and coating density of organic phosphonic acid (PA) modifiers. Single-gas, pressure-decay adsorption measurements showed that with a complete n-butylphosphonic acid (BPA) monolayer, the C3H6/C3H8 kinetic selectivity was > 5 initially, and it approached the equilibrium selectivity of ∼ 1.2 after 20 min, whereas a coating of 4-phosphonobutyric acid (COOHC3PA) with a similar chain length as BPA yielded a selectivity of 15 at 60 min. Coating with 3-aminopropyl phosphonic acid (NH2C3PA) resulted in high resistance to gas diffusion. To investigate whether the slow adsorption was attributable to excessive NH2C3PA, the coating density was tuned by varying PA concentration for deposition. As the coating density decreased, the initial adsorption rates increased. With an ∼ 0.1 monolayer NH2C3PA coating, the C3H6/C3H8 kinetic selectivity was > 15 for 60 min. Temperature-programmed desorption of n-propylamine suggested that the improved selectivity of NH2C3PA coating may be associated with the affinity of the amine group for the zeolite surface. This study demonstrates that gas adsorption rates and selectivities in zeolites are highly sensitive to the composition and density of monolayer films on the external surface.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


