Enhanced fluoride adsorption via coordination tuning in metal–organic frameworks
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
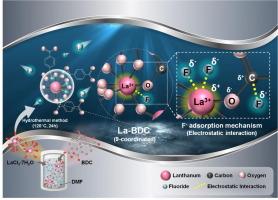
Metal-organic framework (MOF) synthesis with various organic linkers has been reported; however, coordination tuning by changing the central metal has not been well documented. In this study, terephthalic acid (BDC)-type MOFs (La-BDC, Zr-BDC, and Ti-BDC) were prepared by varying the metal precursors to study the effects of the coordination number on the F- adsorption performance. La3+ (9-coordination) resulted in the largest MOF particle size and the highest F- adsorption capacity (163.3 mg g−1). Fourier transform infrared spectroscopy (FTIR) and Raman analyses confirmed higher O-C=O peaks in La-BDC than those in Zr-BDC and Ti-BDC, indicating higher coordination. Time-lapse FTIR, X-ray photoelectron spectroscopy (XPS), and in situ Raman analyses revealed La-O as dominant F- adsorption sites, driven by electrostatic interaction. Selectivity tests with competitive anions such as Cl-, NO3–, and SO42- presented La-BDC’s superior adsorption selectivity for F- uptake. Impressively, La-BDC regeneration with 0.01 M MgCl2 retained 93.6 % F- removal efficiency over five cycles. Its effectiveness across a wide pH range (4.0–10.0) was also confirmed. The column (EBCT = 10 min) test results showed an F- adsorption capacity of 129 mg g−1, which was close to the batch result of 163.3 mg g−1, owing to the minimized diffusion limitation or mass transfer resistance of La-BDC. Economic assessment showed La-BDC synthesis cost and F- adsorption capacity yielded a treatment cost of 720.1 mg F- $-1, lower than UiO-66 (655.2 mg F- $-1). These findings provide a promising alternative approach to improving F- removal by tuning the coordination of MOF.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


