Effect of BaH2 on the hydrogen storage properties of Mg(NH2)2-LiH
IF 5.8
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
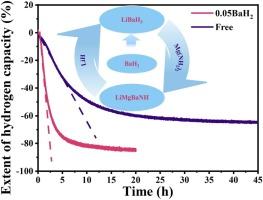
The Mg(NH2)2-2LiH composite has garnered extensive attention on account of its high theoretical hydrogen storage capacity (5.6 wt%) and favorable thermodynamic properties (ca. 40 kJ/mol-H2). However, the sluggish kinetics restricts its practical application. In this study, we investigated in detail the effect of BaH2 doping on the hydrogen absorption and desorption properties of Mg(NH2)2-2LiH. Experimental results demonstrated that the incorporation of BaH2 significantly enhances the hydrogen absorption/desorption kinetic performance compared to the pristine sample. The optimum overall performance was achieved for the sample doped with 0.05 BaH2. Its initial hydrogen release temperature and peak dehydrogenation temperature were decreased by 30 and 20 °C, respectively. The rate of hydrogen release during isothermal dehydrogenation at 160 °C was twice as fast as that of the undoped sample, and the interrelated activation energy was reduced from 112.8 to 72.2 kJ/mol. Additionally, the mechanism underlying improvement of the hydrogen storage performance in the Mg(NH2)2-2LiH composite by BaH2 was elucidated.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


