Synthesis and evolution of 16-membered macrolide carrimycin derivatives as a novel class of anti-HCoV-OC43 agents targeting viral FSE RNA
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
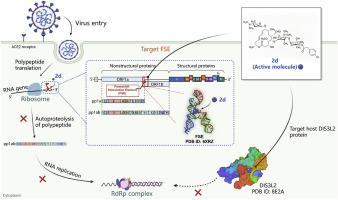
We first demonstrate that carrimycin, as an antibiotic, shows broad-spectrum anti-coronavirus activity by targeting frameshifting element (FSE) RNA. Herein, taking carrimycin as the lead, 26 new 16-membered macrolides were synthesized and evaluated for antiviral activity against coronavirus strains. Compound 2d exhibited the elevated antiviral efficacy against HCoV-OC43 and HCoV-229E with EC50 values of 0.85 μM and 1.45 μM by directly targeting coronaviral FSE RNA pseudoknot. Molecular simulations revealed that the introduction of a 4"-substituent transforms the macrocyclic core into U-shaped conformation, enabling the higher binding with FSE. Meanwhile, using thermal proteome profiling (TPP) technology, we identified DIS3L2 as a potential host target, which probably assisted 2d to exert the antiviral effect. Therefore, the 16-membered macrolides constituted a new class of RNA inhibitors against coronaviruses, and 2d owns a dual-target mechanism that acts on both viral FSE RNA and host DIS3L2.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


