UiO-66 MOFs-Based “Epi-Nano-Sonosensitizer” for Ultrasound-Driven Cascade Immunotherapy against B-Cell Lymphoma
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
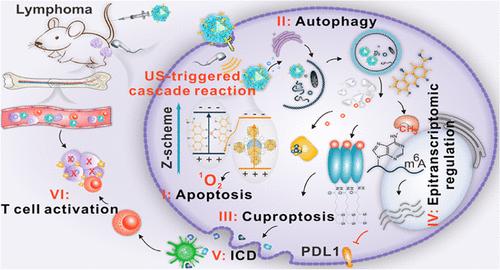
B-cell lymphoma (BCL) is a hematological malignancy with high heterogeneity and represents an aggressive proliferation of mature B-cells. Despite the initial success of traditional treatments for BCL in clinical trials, a majority of patients eventually develop resistance to therapy and have poor clinical outcomes. Epigenetic dysregulation is a major contributor to the pathogenesis of BCL, and therapies targeting epigenetic pathways is a promising alternative strategy for treating BCL. Herein, we developed a metal–organic framework (MOF)-based nano-sonosensitizer for ultrasound-driven cascade immunotherapy against BCL. The nano-sonosensitizer was synthesized by encapsulating copper complex of the m6A-mRNA demethylase inhibitor into UiO-66-NH2, which possesses a Z-scheme heterostructure and allows efficient electron–hole pair separation for generating reactive oxygen species (ROS) under ultrasound activation. These CuR@UiO66 sonosensitizers were functionalized with mPEG-PO3 and anti-CD19 antibody, and the resulting CRUPPA19 particles could specifically accumulate in the BCL tissue and also target lymphoma cells that infiltrated into the bone marrow. Once internalized, CRUPPA19 could induce intracellular ROS production and apoptosis under ultrasound irradiation. Subsequently, ultrasonic stimulation triggered autophagy-mediated release of Cu and Rhein from CRUPPA19, thereby increasing protein lipoylation and global mRNA methylation, which led to cuproptosis and the transcriptional repression PDL1, respectively. These cascades synergistically induced immunogenic cell death in the tumors and promoted activation of CD8+ T cells, eventually leading to an antilymphoma immune response. CRUPPA19-mediated sono-immunotherapy not only eliminated the primary and metastatic lymphomas but also cleared lymphoma cells from the bone marrow. This study provided an insight into a MOF-based nanoepigenetic therapy platform with ultrasound-triggered cascade amplification for enhanced antihematological tumor immunity.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


