MTA-Cooperative PRMT5 Inhibitors: Mechanism Switching Through Structure-Based Design
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Deletion of the MTAP gene leads to accumulation of the substrate of the MTAP protein, methylthioadenosine (MTA). MTA binds PRMT5 competitively with S-adenosyl-l-methionine (SAM), and selective inhibition of the PRMT5•MTA complex relative to the PRMT5•SAM complex can lead to selective killing of cancer cells with MTAP deletion. Herein, we describe the discovery of novel compounds using structure-based drug design to switch the mechanism of binding of known, SAM-cooperative PRMT5 inhibitors to an MTA-cooperative binding mechanism by occupying the portion of the SAM binding pocket in PRMT5 that is unoccupied when MTA is bound and hydrogen bonding to Arg368, thereby allowing them to selectively target MTAP-deleted cancer cells.
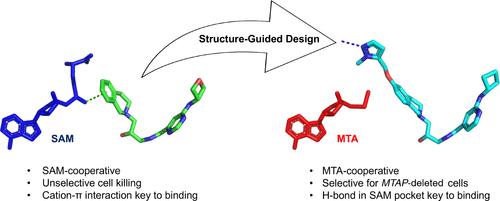
mta协同PRMT5抑制剂:基于结构设计的机制切换
MTAP基因的缺失导致MTAP蛋白底物甲基硫代腺苷(MTA)的积累。MTA将PRMT5与s -腺苷-l-甲硫氨酸(SAM)竞争性结合,相对于PRMT5•SAM复合物,选择性抑制PRMT5•MTA复合物可导致MTAP缺失选择性杀死癌细胞。在此,我们描述了使用基于结构的药物设计的新化合物的发现,通过占据PRMT5中当MTA结合和氢键与Arg368结合时未被占用的SAM结合口袋部分,将已知的SAM协同PRMT5抑制剂的结合机制转换为MTA协同结合机制,从而使它们能够选择性地靶向mtap缺失的癌细胞。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


