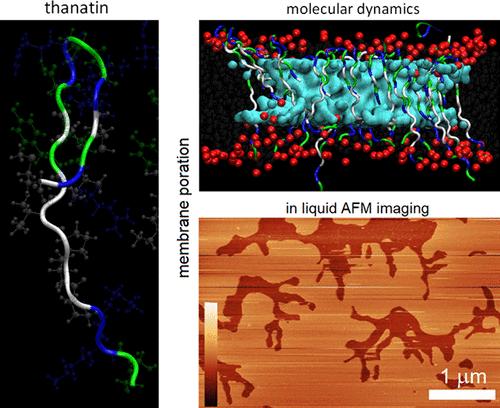
Multimodal Membrane Poration by Thanatin
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Antimicrobial resistance has motivated the search for antimicrobial agents with multimodal mechanisms of action. Host defense peptides and bacteriocins hold particular promise in this regard. Among many molecules discovered to date, thanatin appears to represent the properties of the two classes in that it, like bacteriocins, adopts a highly stable fold in solution and, like host defense peptides, exhibits broad-spectrum antibiotic activity. The peptide is believed to depolarize bacterial outer membranes and inhibit lipopolysaccharide transport while restoring bacterial susceptibility to β-lactam antibiotics. However, a direct observation of whether and how thanatin affects membranes is lacking. Here we reason that the peptide should promote bacteriocin-like multimodal poration in phospholipid bilayers. We demonstrate that thanatin induces poration with elements of membrane thinning, fractal ruptures, and transmembrane channels, a phenomenon common for bacteriocin folds but atypical of antimicrobial peptides. The results offer mechanistic insight into the action of antimicrobial agents emerging from different molecular classes but with similar properties.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


