Synergism of Hydrogen-Induced Interstitial Effect and Gold-Induced Alloying Effect in PdAuH Metallene for Urea Electrosynthesis from Nitrate and Carbon Dioxide
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Urea is a common agricultural fertilizer and industrial raw material, but at present, the traditional industrial production of urea is energy-and pollution-intensive. Electrocatalytic coupling of CO2 and ubiquitous nitrogen sources to synthesize urea is considered as a promising alternative method but requiring high-performance catalysts to boost the C–N coupling electrocatalysis process. Herein, hydrogen-intercalated Pd–Au bimetallene (PdAuHene) was prepared by a three-step method and used for electrosynthesis of urea from NO3– and CO2, deriving an optimum urea Faradaic efficiency of 33.88% and yield rate of 6.68 mmol g–1 h–1 at an applied potential of −0.6 V vs RHE. Detailed material characterizations and electrochemical studies reveal that the metallene structure with ultrathin thickness could improve atomic utilization of precious metal atoms, and the introduction of Au and H atoms could adjust the electronic structure of Pd atoms, regulate the evolution pathway of key N-/C-intermediates, and promote the C–N coupling to form urea.
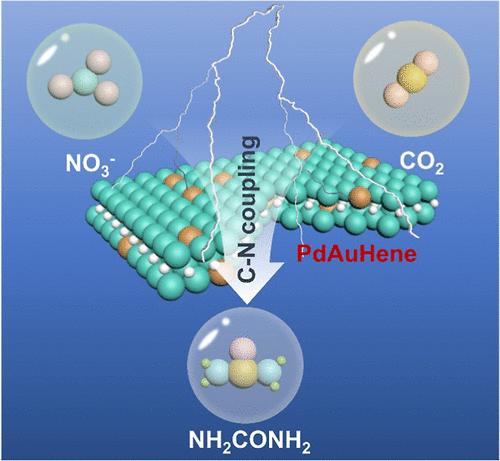
硝酸和二氧化碳电合成尿素中氢致间隙效应和金致合金化效应的协同作用
尿素是一种常见的农业肥料和工业原料,但目前,传统的尿素工业生产是能源和污染密集型的。二氧化碳与氮源电催化偶联合成尿素被认为是一种很有前途的替代方法,但需要高性能的催化剂来促进C-N偶联电催化过程。本文采用三步法制备了插氢Pd-Au双金属烯(PdAuHene),并将其用于NO3 -和CO2电合成尿素,在−0.6 V vs RHE电位下,尿素的最佳法拉第效率为33.88%,产率为6.68 mmol g-1 h-1。详细的材料表征和电化学研究表明,超薄厚度的金属烯结构可以提高贵金属原子的利用率,Au和H原子的引入可以调节Pd原子的电子结构,调节关键N-/ c中间体的演化途径,促进C-N偶联形成尿素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


