In situ polymerization of an electrochemically stable dual-salt gel polymer electrolyte for lithium ion batteries
IF 10.7
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
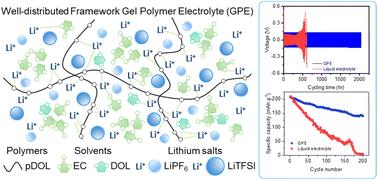
Lithium-ion batteries with solid state electrolytes (SSEs) have improved safety and electrochemical properties; however, the interfacial compatibility between SSEs and electrodes is suboptimal, adversely affecting battery performance. Herein, we report a lithium hexafluorophosphate (LiPF6) and lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) dual salt gel polymer electrolyte (GPE) by in situ polymerization of 1,3-dioxolane (DOL) within a liquid electrolyte composed of an ethylene carbonate environment-friendly solvent. The LiPF6 and LiTFSI concentrations have significant influences on the formation of the interface and the uniformity of composition in the GPE, which affect the degree of polymerization, ionic conductivity, and lithium ion transference number. The optimized GPE (15P15T) exhibits a wide electrochemical stability window up to 5.0 V vs. Li+/Li. Additionally, the Li‖15P15T GPE‖Li cell demonstrates a high stability against the stripping/plating test for over 2000 h at a current density of 1.0 mA cm−2, while the liquid electrolyte (LE) fails from 600 h. The assembled Li‖15P15T GPE‖LiNi0.8Co0.1Mn0.1O2 (NCM811) cell exhibits better cycling performance compared to the Li‖NCM811 cell with the LE. This work provides a reliable approach toward high voltage lithium-ion batteries with enhanced safety and performance.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Materials Chemistry A
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
19.50
自引率
5.00%
发文量
1892
审稿时长
1.5 months
期刊介绍:
The Journal of Materials Chemistry A, B & C covers a wide range of high-quality studies in the field of materials chemistry, with each section focusing on specific applications of the materials studied. Journal of Materials Chemistry A emphasizes applications in energy and sustainability, including topics such as artificial photosynthesis, batteries, and fuel cells. Journal of Materials Chemistry B focuses on applications in biology and medicine, while Journal of Materials Chemistry C covers applications in optical, magnetic, and electronic devices. Example topic areas within the scope of Journal of Materials Chemistry A include catalysis, green/sustainable materials, sensors, and water treatment, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


