Ligand-enabled, Ni-catalyzed dicarbofunctionalization of alkenyl alcohols
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
An alcohol-directed 1,2-dicarbofunctionalization of alkenyl alcohols has been realized with aryl/alkenyl boronic acids and alkyl halides as the coupling partners. This reaction was enabled by a commercially available bulky 3-amyl β-diketone (Amacac) ligand, which enhances the reactivity and suppresses many competitive processes. With alcohol as a weak native directing group, this protocol delivers 1,2-arylalkylated and 1,2-alkenylalkylated alcohols with high efficiency, high regioselectivities, a broad substrate scope, and exceptional functional group tolerance. Notably, this methodology facilitates the modular synthesis of biologically active compounds and key alcohol-containing synthetic intermediates. Preliminary mechanistic studies shed light on the neutral coordination of alcohol functionality to nickel catalysts and the origin of regioselectivity.
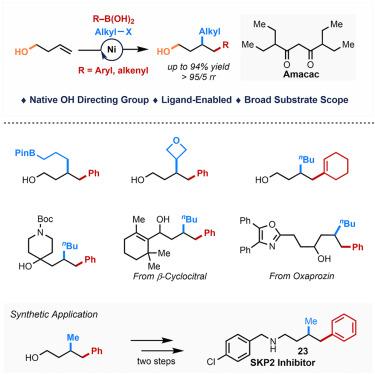
配体激活的镍催化烯醇的二碳功能化
以芳基/烯基硼酸和烷基卤化物为偶联剂,实现了烯基醇定向1,2-二碳功能化反应。这种反应是由一种市售的体积庞大的3-戊基β-二酮(Amacac)配体实现的,它增强了反应活性,抑制了许多竞争过程。由于醇作为弱的天然导向基团,该方案提供1,2-芳基烷基化和1,2-烯基烷基化醇具有高效率、高区域选择性、广泛的底物范围和特殊的官能团耐受性。值得注意的是,这种方法促进了生物活性化合物和关键含醇合成中间体的模块化合成。初步的机理研究揭示了醇官能团对镍催化剂的中性配位和区域选择性的来源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


