Effects of Chelators on Fluoride Removal by AlCl3 and Al13 Coagulation: Performance and Mechanism
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
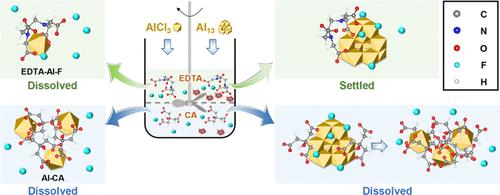
Aluminum (Al) coagulation is one of the most widely used techniques for the defluoridation of industrial effluents. This study examined the influence of two common chelating agents, i.e., ethylenediaminetetraacetic acid (EDTA) and citric acid (CA), on fluoride removal during coagulation using monomeric Al (AlCl3) and polymeric aluminum species [Al13O4(OH)24(H2O)127+, Al13]. The results revealed that EDTA reduced the fluoride removal efficiency by up to 95.4% with AlCl3 and by 28.3% with Al13. In contrast, CA inhibited fluoride removal by up to 100% with AlCl3 and 90.6% with Al13. Both chelators impaired fluoride removal primarily by disrupting floc formation. Advanced analytical techniques, including 19F/27Al NMR spectroscopy combined with DFT calculations, demonstrated that this interference was due to the formation of EDTA-Al-F ternary complexes and Al-CA binary complexes. Additionally, the formation of a bridging fluoride (μ-F) in the Al13 structure enhanced its thermodynamic stability against chelators. Specifically, Al13 maintained structural stability even in the presence of up to 1.50 mmol/L EDTA, whereas CA progressively destabilized the Al13 structure by forming soluble Al-CA complexes. These findings are believed to help guide the rational design of Al-based coagulants and optimize water treatment strategies aimed at defluoridation of industrial effluents.
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


