Fe(II)-Photoantibiotics for Potential Antibacterial, Antibiofilm, and Infective Wound Healing Applications in Rat Model
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
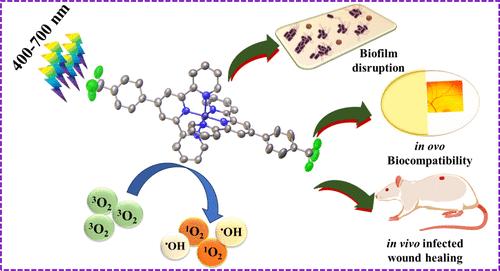
Herein, five Fe(II) complexes, viz., [Fe(N,N,N)2](ClO4)2, where N,N,N = 4′-phenyl-2,2′:6′,2″-terpyridine (Ph-tpy, Fe1), 4′-(4 aminophenyl) 2,2′:6′,2″ terpyridine (NH2-Phtpy, Fe2), 4-([2,2′:6′,2′′-terpyridin]-4′-yl)-N,N-dimethylaniline (NMe2-Phtpy, Fe3), 4′-(p-nitrophenyl)-2.2′:6′,2″-terpyridine (NO2-Phtpy, Fe4), and 4′-(4-trifluoromethylphenyl)-2,2’:6′,2′′-terpyridine (CF3-Phtpy, Fe5) were developed and screened for their visible-light-triggered antibacterial activity. Fe1–Fe5 exhibited absorption at ca. 450–600 nm, beneficial for antibacterial photodynamic therapy (aPDT) under visible light exposure. The excellent photostability and ideal energy gap between T1 and S0 of the complexes made them good photosensitizers for aPDT. Fe5 had the best antibacterial activity against Escherichia coli and Bacillus subtilis upon exposure to 400–700 nm (10 J cm–2) light due to reactive oxygen species (ROS) generation. Further, Fe5 showed antibiofilm activity on different medical-grade biomaterials and devices. Biocompatibility of Fe5 was validated using in vivo and chicken embryonic models (in ovo). Moreover, in vivo studies showed that Fe5 efficiently healed infected wounds within 9 days.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


