Understanding the Influence of Electron Traps and Urbach States on the Kinetics of Ti3+ Persistent Luminescence in LaAlO3:Ti3+
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
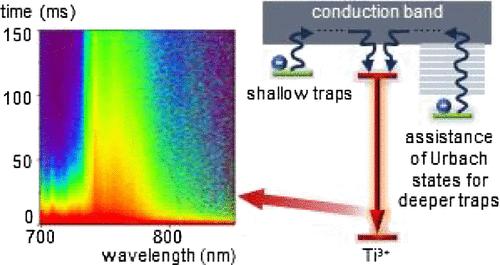
The luminescence kinetics of Ti3+ ions, resulting from the spin-allowed 2E → 2T2 electron transition, are generally expected to be fast, within the microsecond range. However, in this study, we observed average lifetimes of up to 30 ms in nanocrystalline LaAlO3:Ti3+ powders. Our detailed analysis of the spectroscopic and thermoluminescence properties of LaAlO3:Ti3+ suggests that this prolonged Ti3+ kinetics is associated with the presence of electron traps and the proximity of the Ti3+ excited state to the conduction band, which facilitates energy transfer between them. Furthermore, the observed shift in Urbach states with an increasing Ti3+ concentration correlates with the efficiency of energy transfer between deeper traps and Ti3+ ions. This study provides a comprehensive strategy for controlling the luminescence kinetics of Ti3+ ions through electron trap engineering, induced by dopant ion concentration, which can be applied in various fields including luminescence thermometry.
LaAlO3:Ti3+中电子陷阱和Urbach态对Ti3+持续发光动力学的影响
Ti3+离子的发光动力学是由自旋允许的2E→2T2电子跃迁引起的,通常预计会很快,在微秒范围内。然而,在这项研究中,我们观察到纳米晶LaAlO3:Ti3+粉末的平均寿命高达30 ms。我们对LaAlO3:Ti3+的光谱和热释光特性的详细分析表明,这种延长的Ti3+动力学与电子陷阱的存在和Ti3+激发态接近导带有关,这有利于它们之间的能量转移。此外,随着Ti3+浓度的增加,Urbach态的位移与更深的陷阱和Ti3+离子之间的能量转移效率有关。本研究提供了一种通过掺杂离子浓度诱导的电子陷阱工程来控制Ti3+离子发光动力学的综合策略,可应用于包括发光测温在内的各个领域。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


