Stepwise H*-Mediated and Non-H* Reduction Processes for Highly Selective Transformation of Nitrate to Nitrogen Gas Using a ZVAl-Based Material
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Improving the reduction efficiency and N2 selectivity is important for nitrate decontamination. A novel ternary ball-milled Al-Cu-AC material is reported to achieve a highly selective reduction of nitrate to N2. The reduction process, driven by the continuous dissolution of zero-valent aluminum (ZVAl), demonstrated a stepwise reduction scheme. The interesting shift in the electron-donating pathways was ascribed to the spontaneous change in the microenvironmental pH from neutral to alkaline. The Al-Cu-AC (1:1:5 mass ratio) material completely removed 30 mg/L of NO3–-N over a wide pH range (5–9), achieving over 83% TN removal and N2-selectivity, without detectable copper leaching. The atomic hydrogen (H*)-mediated reduction occurring on the Cu component was proven to be crucial for the fast transformation from NO3– to NO2–, while the non-H* reduction process was dominated by the electrochemical reduction of NO2– to N2 on the AC cathode of Al || AC microgalvanic cells formed in the material. The primary reduction route from NO3– to N2 was identified as the *NOH pathway, and the superiority of the Al-Cu-AC material toward nitrate reduction was verified with actual wastewater. This study revealed how microenvironmental pH influenced the electron-donating pathways of ZVAl and provides a new approach to maximize the performance of zero-valent metals.
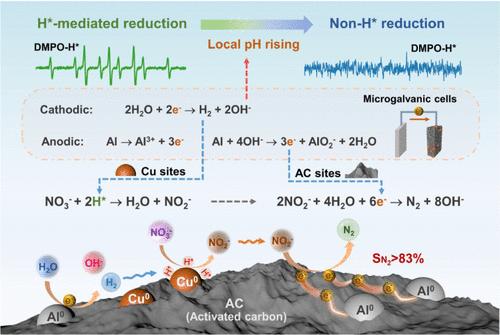
利用zval基材料,逐步H*介导和非H*还原过程高选择性将硝酸盐转化为氮气
提高还原效率和N2选择性对硝酸盐净化具有重要意义。一种新型的三元球磨Al-Cu-AC材料实现了硝酸还原为N2的高选择性。在零价铝(ZVAl)连续溶解的驱动下,还原过程呈现出逐步还原的模式。给电子途径的有趣转变归因于微环境pH从中性到碱性的自发变化。Al-Cu-AC(1:1:5质量比)材料在较宽的pH范围内(5-9)完全去除30 mg/L的NO3—N,达到83%以上的TN去除率和n2选择性,没有检测到铜浸出。Cu组分上发生的原子氢(H*)介导的还原是NO3 -快速转化为NO2 -的关键,而非H*还原过程主要是在材料中形成的Al ||交流微原电池的交流阴极上由NO2 -电化学还原为N2。确定了NO3 -还原为N2的主要途径为*NOH途径,并通过实际废水验证了Al-Cu-AC材料还原硝酸盐的优越性。该研究揭示了微环境pH对ZVAl给电子途径的影响,为零价金属性能的最大化提供了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


