The Role of Natural Organic Matter in the Degradation of Phenolic Pollutants by Sulfate Radical Oxidation: Radical Scavenging vs Reduction
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
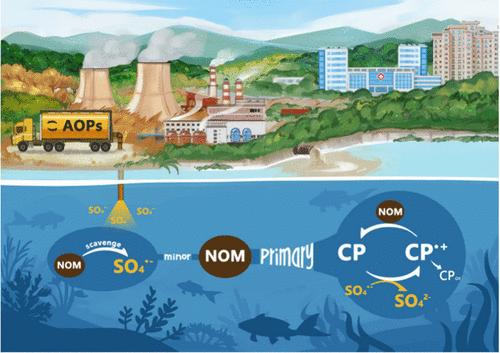
Dissolved natural organic matter (NOM) significantly influences the performance of water treatment processes. It is generally recognized that NOM acts as a radical scavenger, thus inhibiting the degradation of organic pollutants in advanced oxidation processes (AOPs). This study examined the impacts of 8 different NOM isolates on the degradation of 4-chlorophenol (CP), a representative phenolic pollutant, in sulfate radical (SO4•–)-based AOPs. We developed an improved probe method to measure the steady-state concentration of SO4•– ([SO4•–]ss) in both the absence and presence of NOM. Results show that adding 1.00 mgC L–1 NOM resulted in only a 1.3–3.4% decrease in [SO4•–]ss. However, the apparent rate constants of CP degradation decreased by 76–88%. This discrepancy indicates that radical scavenging cannot be the primary mechanism for observed inhibition. We proposed NOM primarily acts as a reducing agent, reacting with the phenoxy radical intermediates generated from the single-electron oxidation of CP by SO4•–. Based on this hypothesis, we developed and validated a kinetic model using experimental data. The reductive capacity of NOM, as determined by the kinetic model, correlates positively with its electron-donating capacity. These findings enhance the understanding of NOM’s role in SO4•–-based AOPs and provide a foundation for developing strategies to mitigate its adverse effects.
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


