Self-Assembled arachidonic acid Nanomicelles for Radiation-Induced ferroptosis to enhance tumor Radioimmunotherapy
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
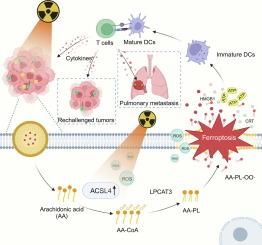
Radiotherapy (RT) induces cancer cell death primarily through apoptosis, a non-inflammatory process that triggers a limited antitumor immune response, making it insufficient to prevent tumor metastasis and recurrence. Ferroptosis is a form of cell death caused by the peroxidation of phospholipids (PLs) containing polyunsaturated fatty acid (PUFA) in the cell membrane. It has been reported that RT can induce ferroptosis, but the low PUFA content in tumors limits the ability of radiation to effectively induce ferroptosis. Herein, we designed a self-assembled nanomicelle (ADM) to deliver arachidonic acid (AA, a type of PUFA) to enhance radiation-induced ferroptosis. Radiation can upregulate acyl-CoA synthetase long chain family member 4 (ACSL4), which converts the abundant AA taken up by cancer cells into AA-PLs and incorporates them into cell membrane. More importantly, reactive oxygen species (ROS) generated by RT promote the peroxidation of PLs containing high levels of PUFA in the cell membrane, thereby inducing ferroptosis and subsequently triggering an effective antitumor immune response. Moreover, encapsulating the Toll-like receptor agonist resiquimod in ADM further potentiates systemic antitumor immunity, significantly inhibiting lung metastasis of 4 T1 breast cancer and preventing tumor rechallenge. Our work presents a new strategy to enhance RT-induced ferroptosis by developing a nanoplatform capable of delivering PUFA.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


