Discovery of Naphthoquinone-Furo-Piperidone Derivatives as Dual Targeting Agents of STAT3 and NQO1 for the Treatment of Breast Cancer
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
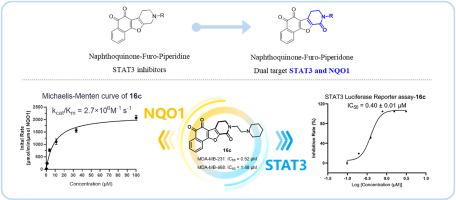
Breast cancer is one of the most common malignancies in women, posing a significant threat to their physical and mental well-being. STAT3 has been found closely associated with the occurrence and development of breast cancer, while blocking STAT3 pathway can promote apoptosis of breast cancer cells and inhibit cell proliferation. NQO1 is a potential anti-tumor drug target, and its substrate has been widely proven to show significant anti-tumor activity. Thus, those agents that could simultaneously target STAT3 and NQO1 might provide a new approach for the treatment of breast cancer. Herein, we have designed and synthesized novel naphthoquinone-furo-piperidone derivatives as dual targeting agents of STAT3 and NQO1. The anti-proliferative activity evaluation revealed that most of these compounds exhibited superior inhibitory activity against MDA-MB-231 and MDA-MB-468 breast cancer cell lines compared to napabucasin. In particular, the promising compound 16c was found to significantly inhibit phosphorylation of STAT3 at Tyr705 at a concentration of 1 μM and effectively induce apoptosis in MDA-MB-231 and MDA-MB-468 breast cancer cells. Moreover, 16c was also found as a NQO1 substrate to strongly increase ROS generation and cause severe DNA damage in a dose-dependent manner. Meanwhile, 16c showed encouraging anti-tumor efficacy in the MDA-MB-231 xenograft model. In summary, this protocol provides a new vision and new chemical entity for dual targeting STAT3 and NQO1.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


