Nitric Oxide-Producing Multiple Functional Nanoparticle Remodeling Tumor Microenvironment for Synergistic Photodynamic Immunotherapy against Hypoxic Tumor
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The treatment of pancreatic cancer faces significant challenges due to connective tissue hyperplasia and severe hypoxia. Unlike oxygen-dependent Type II photosensitizers, Type I photosensitizers can produce a substantial amount of reactive oxygen species, even under hypoxic conditions, making them more suitable for photodynamic therapy of pancreatic cancer. However, the dense extracellular matrix of pancreatic cancer limits the penetration efficiency of photosensitizers, and the presence of immunosuppressive cells in the tumor microenvironment reduces the therapeutic effect. To address these challenges, we designed the photoimmunotherapeutic M1@PAP nanoparticles composed of Type I photosensitizer and anti-PD-L1 siRNA (siPD-L1), which was encapsulated into M1 macrophage membrane vesicles. In this system, pyropheophorbide-a (PPA) was covalently conjugated to poly-l-arginine (Arg9). Notably, it was capable of generating sufficient superoxide anions under hypoxic conditions, thereby functioning as a Type I photosensitizer. Furthermore, Arg9 acted as a nitric oxide (NO) donor, enhancing the penetration efficiency of the nanophotosensitizer by inhibiting cancer-associated fibroblast (CAF) activation and decomposing the tumor extracellular matrix. Additionally, M1 macrophage membrane vesicles provided active targeting capabilities and reeducated immunosuppressed M2 macrophages. The reversal of immunosuppressive microenvironment further promoted the efficacy of anti-PD-L1 siRNA immunotherapy, showing great potential in synergistic photodynamic immunotherapy against hypoxic pancreatic tumor.
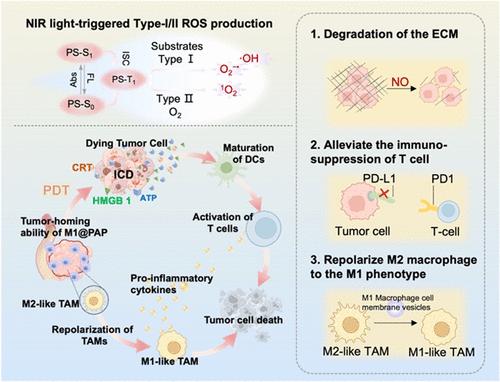
产生一氧化氮的多功能纳米颗粒重塑肿瘤微环境用于协同光动力免疫治疗缺氧肿瘤
由于结缔组织增生和严重缺氧,胰腺癌的治疗面临重大挑战。与依赖氧的II型光敏剂不同,I型光敏剂即使在缺氧条件下也能产生大量的活性氧,使其更适合于胰腺癌的光动力治疗。然而,胰腺癌致密的细胞外基质限制了光敏剂的穿透效率,肿瘤微环境中免疫抑制细胞的存在降低了治疗效果。为了解决这些挑战,我们设计了由I型光敏剂和抗pd - l1 siRNA (siPD-L1)组成的光免疫治疗M1@PAP纳米颗粒,并将其包裹在M1巨噬细胞膜囊泡中。在该体系中,焦磷-a (PPA)与聚l-精氨酸(Arg9)共价偶联。值得注意的是,它能够在缺氧条件下产生足够的超氧阴离子,从而作为I型光敏剂发挥作用。此外,Arg9作为一氧化氮(NO)供体,通过抑制癌症相关成纤维细胞(CAF)的激活和分解肿瘤细胞外基质,提高纳米光敏剂的渗透效率。此外,M1巨噬细胞膜囊提供了主动靶向能力和再教育免疫抑制的M2巨噬细胞。免疫抑制微环境的逆转进一步促进了抗pd - l1 siRNA免疫治疗的疗效,在缺氧胰腺肿瘤的协同光动力免疫治疗中显示出巨大的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


