Small circular RNAs as vaccines for cancer immunotherapy
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
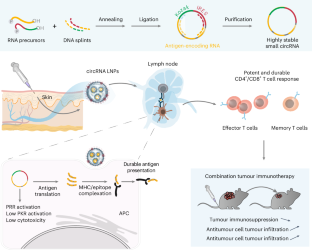
Messenger RNA vaccines have shown strong prophylactic efficacy against viral infections. Here we show that antigen-encoding small circular RNAs (circRNAs) loaded in lipid nanoparticles elicit potent and durable T cell responses for robust tumour immunotherapy after subcutaneous injection in mice, particularly when combined with immune checkpoint inhibition. The small circRNA vaccines are highly stable and show low levels of activation of protein kinase R as well as low cytotoxicity, enabling long-lasting antigen translation (longer than 1 week in cells). Relative to large protein-encoding unmodified or modified mRNAs and circRNAs, small circRNA vaccines elicited up to 10-fold antigen-specific T cells in mice and accounted for 30–75% of the total peripheral CD8+ T cells over 6 months. Small circRNA vaccines encoding tumour-associated antigens, neoantigens and oncoviral or viral antigens elicited substantial CD8+ and CD4+ T cell responses in young adult mice and in immunosenescent aged mice. Combined with immune checkpoint inhibition, monovalent and multivalent circRNA vaccines reduced tumour-induced immunosuppression and inhibited poorly immunogenic mouse tumours, including melanoma resistant to immune checkpoint blockade. Small circular RNAs encoding one or more antigens and enclosed in subcutaneously injected lipid nanoparticles can elicit potent and durable antitumour T cell responses for robust tumour immunotherapy, particularly in combination with immune checkpoint inhibition, as shown in multiple mouse models of tumours.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


