Regulation of Reaction Pathways in Coordinated Chains by Directional Mechanical Force
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
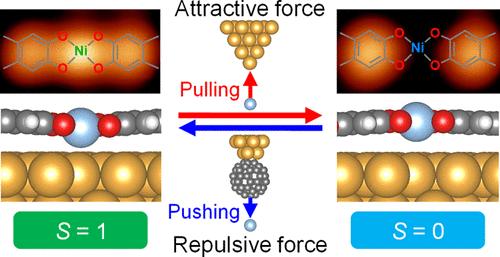
Mechanochemistry refers to chemical reactions induced by mechanical forces. Due to different reaction mechanisms, products obtained through mechanochemistry can be distinct from those produced by thermochemistry and photochemistry. Scanning probe microscopy is a powerful tool for studying single-molecule mechanochemical processes. Mechanical force is a vector that has both magnitude and direction. Previous studies have focused on triggering reactions by forces and measuring their magnitude. In this work, we use the direction of the force to regulate the reaction pathway in a spin-crossover coordinated chain. The chains are prepared via the dehydrogenated coordination reaction between tetrahydroxybenzene molecules and Ni atoms on Au(111). The Ni atoms in the chain alternate between a high-spin state and a low-spin state. By altering Ni–O bond lengths and O–Ni–O angles through the directional mechanical force, a chemical process occurs, and the spin state of Ni undergoes a transition. With the attraction from a Au tip, the Ni atom is pulled from high-spin to low-spin state. With the repulsion from a C60-functionalized tip, the low-spin Ni atom is pushed to the high-spin state. The force to induce the reaction is measured by qPlus atomic force microscopy. This study provides an approach for regulating chemical pathways.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


