Ionizable Lipids with Optimized Linkers Enable Lung-Specific, Lipid Nanoparticle-Mediated mRNA Delivery for Treatment of Metastatic Lung Tumors
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Lipid nanoparticles (LNPs) have emerged as a groundbreaking delivery system for vaccines and therapeutic mRNAs. Ionizable lipids are the most pivotal component of LNPs due to their ability to electrostatically interact with mRNA, allowing its encapsulation while concurrently enabling its endosomal escape following cellular internalization. Thus, extensive research has been performed to optimize the ionizable lipid structure and to develop formulations that are well tolerated and allow efficient targeting of different organs that result in a high and sustained mRNA expression. However, one facet of the ionizable lipids’ structure has been mostly overlooked: the linker segment between the ionizable headgroup and their tails. Here, we screened a rationally designed library of ionizable lipids with different biodegradable linkers. We extensively characterized LNPs formulated using these ionizable lipids and elucidated how these minor structural changes in the ionizable lipids structure radically influenced the LNPs’ biodistribution in vivo. We showed how the use of amide and urea linkers can modulate the LNPs’ pKa, resulting in an improved specificity for lung transfection. Finally, we demonstrated how one of these lipids (lipid 35) that form LNPs entrapping a bacterial toxin [pseudomonas exotoxin A (mmPE)] in the form of an mRNA reduced tumor burden and significantly increased the survival of mice with lung metastasis.
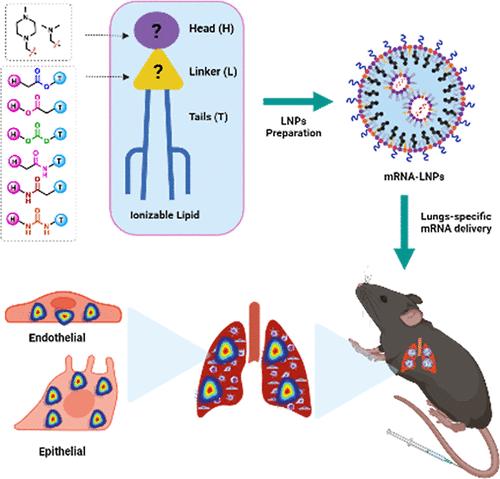
脂质纳米颗粒(LNPs)已成为疫苗和治疗用 mRNA 的突破性传输系统。可电离脂质是 LNPs 最关键的成分,因为它们能与 mRNA 发生静电相互作用,在将其包裹的同时,还能使其在细胞内化后逃逸到内体。因此,人们进行了大量研究,以优化可离子化的脂质结构,并开发出耐受性良好、可有效靶向不同器官的制剂,从而实现 mRNA 的高水平持续表达。然而,可电离脂质结构的一个方面大多被忽视,即可电离头基和尾基之间的连接段。在此,我们筛选了一个合理设计的可离子化脂类库,其中含有不同的可生物降解连接体。我们对使用这些可离子化脂质配制的 LNPs 进行了广泛的表征,并阐明了可离子化脂质结构中的这些微小结构变化是如何从根本上影响 LNPs 在体内的生物分布的。我们展示了使用酰胺和脲连接体如何调节 LNPs 的 pKa,从而提高肺部转染的特异性。最后,我们展示了其中一种脂质(脂质 35)是如何形成以 mRNA 形式夹带细菌毒素[假单胞菌外毒素 A (mmPE)]的 LNPs,从而减轻肿瘤负担并显著提高肺转移小鼠的存活率的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


