Dynamic Regeneration of Catalytic Sites on V-Modified NiCo Oxide for Boosting Urea Electrooxidation
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Nickel-based electrocatalysts have emerged as promising candidates for the urea oxidation reaction (UOR) in alkaline media. However, their active site availability is hindered by catalyst dissolution and inadequate surface active sites during the reaction. Here, we report the construction of stable high-valence V sites in a nickel cobalt vanadate catalyst (NiCoVOx) for boosted UOR performance. After prolonged urea electrolysis, the catalyst structure and composition remain stable with suppressed V dissolution. In situ spectroscopies reveal that the V atoms directly act as active sites to interact with OH– alongside the urea adsorption sites on Ni, which facilitate the C–N cleavage step of urea dehydrogenation. Accordingly, the Ni–V dual sites lead to accelerated UOR kinetics and stabilized valence state of Ni, thereby providing dynamically available active and UOR selective sites. As a result, the UOR on NiCoVOx is obviously promoted with a 460 mV lower overpotential at 100 mA cm–2 and 88% Faradaic efficiency for nitrogen-containing products. This work provides insights into the rational design of catalysts for multielectron small molecule electrooxidation reactions.
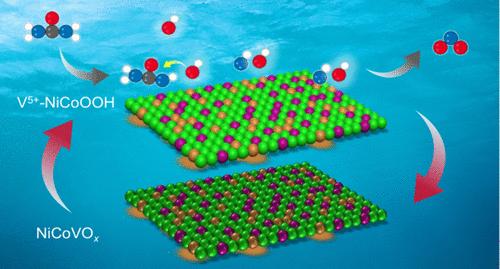
v改性NiCo氧化物催化位点的动态再生促进尿素电氧化
镍基电催化剂已成为尿素氧化反应(UOR)的理想催化剂。然而,在反应过程中,由于催化剂溶解和表面活性位点不足,它们的活性位点可用性受到阻碍。在这里,我们报道了在钒酸镍钴催化剂(NiCoVOx)中构建稳定的高价V位点以提高UOR性能。经过长时间的尿素电解,催化剂的结构和组成保持稳定,抑制了V的溶解。原位光谱分析表明,V原子与Ni上的尿素吸附位点直接作为活性位点与OH -相互作用,促进了尿素脱氢的C-N裂解步骤。因此,Ni - v双位点加速了UOR动力学,稳定了Ni的价态,从而提供了动态可用的活性位点和UOR选择性位点。结果,NiCoVOx上的UOR明显提高,在100 mA cm-2下过电位降低460 mV,含氮产物的法拉第效率为88%。本研究为多电子小分子电氧化反应催化剂的合理设计提供了新的思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


