Amides Enable Room-Temperature CO2 Conversion: Simple Organic Molecules Challenging Metal Catalysts
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
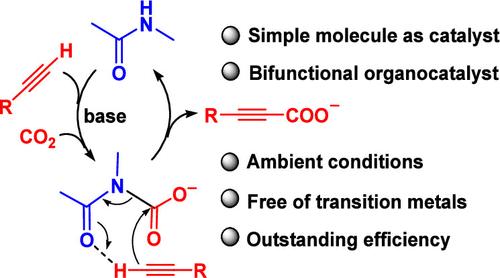
The conversion of carbon dioxide (CO2) into valuable chemicals has been intensively pursued for sustainable chemistry. It is highly desirable to achieve the conversion under ambient conditions using organocatalysts instead of precious or pollutive metal catalysts. Herein, we disclose a new class of organocatalysts for direct C(sp)–H carboxylation with CO2. Amide molecules such as N-methylacetamide and valerolactam behave as efficient bifunctional catalysts to promote the conversion of aromatic alkynes to propiolic acids. In particular, the simple organic catalysts enable the reaction to occur at room temperature, which has been achieved only with complex transition metal catalysts prior to this report. In the presence of the optimal base of Cs2CO3, the adjacent nitrogen and oxygen sites of the amide group concurrently activate CO2 and C(sp)–H and position them in favor of C–C coupling, affording a high catalytic activity on par with those of transition metal catalysts. The work sheds new light on the catalytic chemistry of CO2 and also illustrates the great potential of discovering new organocatalysts from simple molecules.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


