Electrochemical Synthesis of 2-Amino-1,3-benzoxazines via TBAI-mediated Desulfurative Cyclization of Isothiocyanates and 2-Aminobenzyl Alcohols
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
An efficient one-step protocol has been developed to access a variety of 2-amino-1,3-benzoxazine derivatives via tetrabutylammonium iodide-mediated electrochemical desulfurative cyclization of isothiocyanates and 2-aminobenzyl alcohols. The reaction proceeds through a cycle involving in situ iodine generation, desulfurative cyclization, and iodide regeneration, efficiently forming intermolecular C–O and C–N bonds and affording 2-amino-1,3-benzoxazines in moderate to excellent yields. The practical utility of this strategy is evidenced by its broad substrate scope, good functional group compatibility, scalability to gram-scale synthesis, and metal- and oxidant-free conditions.
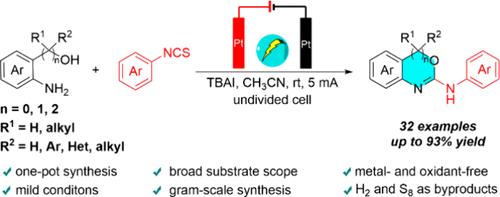
异硫氰酸酯和2-氨基苄醇脱硫环化电化学合成2-氨基-1,3-苯并恶嗪
通过四丁基碘化铵介导的异硫氰酸酯和2-氨基苄醇的电化学脱硫环化,开发了一种高效的一步法来获得各种2-氨基-1,3-苯并恶嗪衍生物。该反应经过一个包括原位碘生成、脱硫环化和碘化物再生的循环,有效地形成分子间的C-O和C-N键,并以中等到优异的收率提供2-氨基-1,3-苯并恶嗪。该策略的实际应用证明了其广泛的底物范围,良好的官能团相容性,可扩展到克级合成,以及无金属和无氧化剂的条件。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


