Efficient and modular synthesis of ibogaine and related alkaloids
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
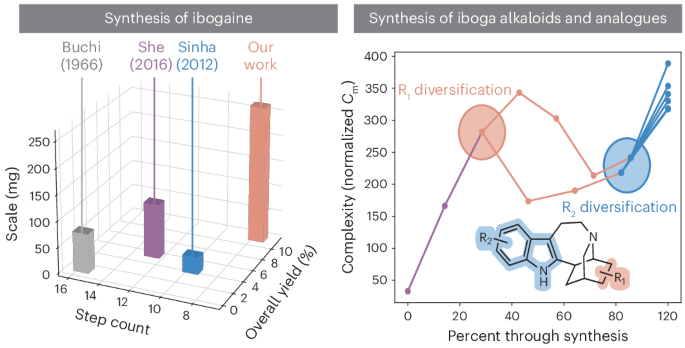
Anecdotal reports and preliminary clinical trials suggest that the psychoactive alkaloid ibogaine and its active metabolite noribogaine have powerful anti-addictive properties, producing long-lasting therapeutic effects across a range of substance use disorders and co-occurring neuropsychiatric diseases such as depression and post-traumatic stress disorder. Here we report a gram-scale, seven-step synthesis of ibogaine from pyridine. Key features of this strategy enabled the synthesis of three additional iboga alkaloids, as well as an enantioselective total synthesis of (+)-ibogaine and the construction of four analogues. Biological testing revealed that the unnatural enantiomer of ibogaine does not produce ibogaine-like effects on cortical neuron growth, while (−)-10-fluoroibogamine exhibits exceptional psychoplastogenic properties and is a potent modulator of the serotonin transporter. This work provides a platform for accessing iboga alkaloids and congeners for further biological study. Preliminary clinical trials suggest that ibogaine and its active metabolite noribogaine have powerful anti-addictive properties, Now, a strategy for the scalable, asymmetric total synthesis of ibogaine has been developed that also provides access to iboga analogues. Biological testing identified a psychoplastogenic iboga analogue that is a potent modulator of the serotonin transporter.

高效模块化合成伊博格碱及相关生物碱
轶事报告和初步临床试验表明,精神活性生物碱伊博格碱及其活性代谢物去甲博格碱具有强大的抗成瘾特性,对一系列物质使用障碍和同时发生的神经精神疾病(如抑郁症和创伤后应激障碍)产生持久的治疗效果。在这里,我们报道了一个克级的,由吡啶合成伊博格碱的七个步骤。该策略的主要特点是合成了三种额外的伊博加生物碱,以及(+)-伊博加碱的对映选择性全合成和四种类似物的构建。生物学测试表明,伊博格碱的非天然对映体不会对皮质神经元生长产生类似伊博格碱的作用,而(−)-10-氟伊博格碱表现出特殊的心理可塑性,是一种有效的5 -羟色胺转运体调节剂。这项工作为进一步研究伊博加生物碱及其同源物提供了平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


