Unlocking Efficient Electrosynthesis of α‐Amino Acids: Adsorption Geometry Modulation and Electronic Structure Reconstruction in the Ag/Cu Bimetallic System
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
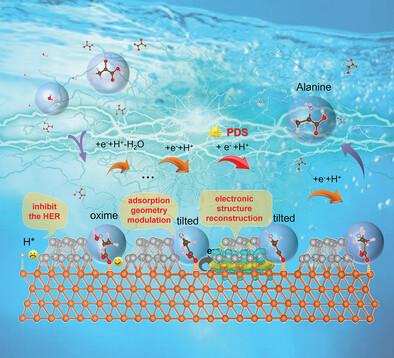
Electrosynthesis of α‐amino acids from α‐keto acids is a promising strategy but faces challenges such as high reduction potential and limited efficiency due to sluggish reaction kinetics and competitive side reactions. Here, this study presents a bimetallic Ag/Cu nanowires (NWs) catalyst that effectively addresses these issues, demonstrating an exceptionally low onset‐potential of −0.18 V versus RHE for alanine electrosynthesis and achieving a remarkable alanine yield of 690 µmol h
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


