Molecular Dynamics Simulations of Interfacial Tensions and Contact Angles of the Nitrogen+Oil+Brine+Rock System
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
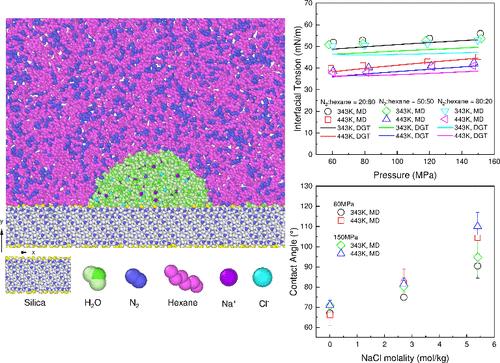
Molecular dynamics (MD) simulations of the N2+hexane+H2O system (two-phase) were conducted at 343–443 K and 60–150 MPa. The MD results agreed reasonably well with the density gradient theory (DGT) results based on the CPA EoS. The interfacial tensions (IFTs) were found to increase with pressure and decrease with temperature. An important finding is that the IFTs only slightly decreased with increasing N2 mole fraction in the N2/hexane-rich phase (xN2). In general, N2 shows a positive surface excess, and hexane shows a negative surface excess. The increase in the IFT with pressure indicates that the IFT behavior is dominated by the negative surface excess of hexane. MD simulations of the corresponding N2+hexane+H2O+silica (hydrophilic) system showed that the water contact angles (CAs) are not greatly affected by pressure or temperature. Importantly, the water CAs slightly decreased with increasing xN2, and the adhesion tensions increased with increasing xN2. MD simulations of the N2+hexane+brine system were also conducted (salt concentration (cs) up to 5.4 mol/kg NaCl). The MD results agreed reasonably well with the DGT results based on the CPA EoS with the Debye–Hückel contribution. Here, Na+ and Cl– were excluded from the interfacial regions. The solubility of N2 in the H2O-rich phase decreased with increasing cs, because of the salting-out effect. The IFTs increased linearly with increasing cs. MD simulations of the corresponding N2+hexane+brine+silica (hydrophilic) system showed that the water CAs increase with increasing cs. Our previous studies showed that the CO2+hexane and CO2+hexane+silica (hydrophilic) systems in the presence of water or brine gave generally similar results. However, for example, the adhesion tensions of the CO2+hexane+H2O+silica (hydrophilic) system decreased with increasing xCO2.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


