Green chemistry approaches in the analytical validation of fosravuconazole using UV spectrophotometry and HPLC
IF 6.2
引用次数: 0
Abstract
Fosravuconazole, a newly developed oral antifungal medication by Eisai, has been utilized since 2018 for the treatment of tinea unguium. Its notable characteristics include high oral absorption and systemic bioavailability, which allow for a brief treatment duration of 3 months. Its mild inhibition of the cytochrome P450 enzyme, which plays a role in adverse effects during polypharmacy, allows for the safe use of fosravuconazole alongside other medications, making it suitable for elderly patients. This study addresses the need for a precise quantitative method to determine “Fosravuconazole” using UV spectrophotometry and High-Performance Liquid Chromatography (HPLC). The HPLC method used an isocratic approach with a reversed-phase CHROMASIL C18 column (4.6 mm × 250 mm, 5 µm), a flow rate of 0.9 mL/min and detection at a wavelength of 287 nm. The mobile phase was a mixture of Acetonitrile and a 10 mM Ammonium Acetate buffer at pH 4.5, with the pH adjusted using acetic acid. Both methods were rigorously validated according to ICH Q2(R1) guidelines, demonstrating their suitability for the assessment of individual substances in various mixtures. To evaluate their environmental impact, AGREE, GAPI, and BAGI were used to evaluate the methods highlighting their sustainability in terms of solvent consumption, chemical and energy use, waste generation. BAGI showed that both methods possess scores above the recommended threshold score of 60, qualifying them for industrial applications. The UV method demonstrated a greener profile compared to the HPLC method.
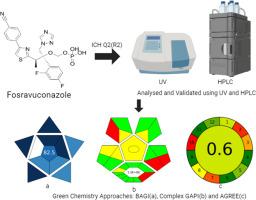
紫外分光光度法和高效液相色谱法在非曲康唑分析验证中的绿色化学方法
卫材新开发的口服抗真菌药物Fosravuconazole从2018年开始用于治疗甲癣。其显著特点是口服吸收和全身生物利用度高,治疗时间短,仅需3个月。它对细胞色素P450酶有轻微的抑制作用,而细胞色素P450酶在多药过程中起不良反应,这使得fosravuconazole与其他药物一起安全使用,使其适合老年患者。本研究探讨了采用紫外分光光度法和高效液相色谱法测定“非司伐康唑”的精确定量方法。HPLC法采用等密度法,色谱柱为CHROMASIL C18反相柱(4.6 mm × 250 mm, 5µm),流速为0.9 mL/min,检测波长为287 nm。流动相为乙腈和10 mM醋酸铵缓冲液的混合物,pH为4.5,用乙酸调节pH。这两种方法都根据ICH Q2(R1)指南进行了严格验证,证明了它们对各种混合物中单个物质评估的适用性。为了评估其对环境的影响,使用了AGREE、GAPI和BAGI来评估在溶剂消耗、化学和能源使用、废物产生方面突出其可持续性的方法。BAGI表明,这两种方法的得分都高于60分的推荐阈值,使它们有资格用于工业应用。与高效液相色谱法相比,紫外法更环保。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


