Structure–Activity Relationship Studies of DNA Methyltransferase 1 Monovalent Degraders
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
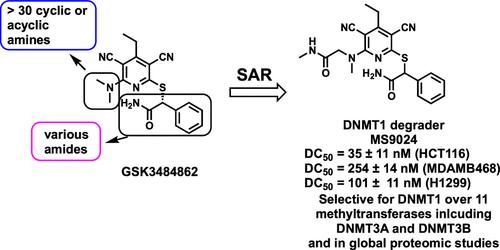
DNA methyltransferase 1 (DNMT1), which catalyzes maintenance methylation of hemimethylated DNA during DNA replication, is overexpressed in cancer. Recently, the first-in-class DNMT1-selective noncovalent small-molecule inhibitors, GSK3484862 and GSK3685032, were discovered. These inhibitors were also reported to degrade DNMT1. However, structure–activity relationship (SAR) studies of these monovalent DNMT1 degraders are lacking. Here, we report our SAR studies of this scaffold on degrading DNMT1, which led to the discovery of multiple lead degraders, including compound 4 (MS9024). Compound 4 potently and selectively degraded DNMT1 in multiple cancer cell lines in a concentration-, time-, and proteasome-dependent manner without altering DNMT1 transcription. Further mechanism-of-action studies suggest that the DNMT1 degradation induced by 4 was not mediated by lysosome or cullin RING E3 ligases but could potentially be mediated by HECT E3 ligases and/or UHRF1. Collectively, these studies pave the way for further developing DNMT1 monovalent degraders as potential therapeutics and useful chemical tools.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


