A Ketone Synthesis via Cu(I)-Catalyzed Regioselective Coupling of 2-Pyridylthioesters with Grignard Reagents: In Quest of Straightforward Access to Pharmaceuticals
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
For commercial production of SGLT2 inhibitors, cryogenic conditions (−78 °C) were required at the key C-glycosidation step, which has restricted their supply to the world market. To address the challenge, reported herein is a new synthetic method based on a new ketone synthesis by means of copper(I)-catalyzed coupling of 2-pyridylthioesters with Grignard reagents. The facile transformation from the ketones to the final APIs was realized under mild conditions due to the use of readily cleavable acetyl protecting groups.
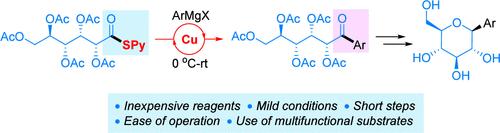
通过铜(I)催化2-吡啶基硫酯与格氏试剂的区域选择性偶联合成酮:寻求直接获得药物的途径
对于SGLT2抑制剂的商业化生产,在关键的C-糖苷化步骤中需要低温条件(- 78°C),这限制了它们在世界市场的供应。为了解决这一挑战,本文报道了一种基于铜(I)催化2-吡啶基硫酯与格氏试剂偶联合成酮的新方法。由于使用了易于切割的乙酰基保护基团,在温和的条件下实现了从酮到最终原料药的容易转化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


