Unleashing Selective Reduction and Reductive Methylation of N-Heterocycles Using Methanol via Strategic Reaction Condition Modulation
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Saturated N-heterocycles and the N-methylated or alkylated derivatives are vital in different areas of science due to their diverse biological and pharmacological activities. Thus, achieving their selective formations is highly essential yet demands innovative approaches. We here reported an Ir(III)-catalyzed selective reduction and N-methylation of N-heterocycles using methanol as a dual H2 and methylating source. The selectivity was precisely governed through strategic modulation of reaction parameters, where AgOTf was essential for the reduction, and 2,2,2-trifluoroethanol (TFE) facilitated the reductive methylation. The methodology was also successfully extended to other alcohols for the reductive alkylation reaction as well as the synthesis of several molecules of various biological importance. Control experiments, kinetic studies, and density functional theory (DFT) calculations further revealed an Ir(III)-catalyzed outer-sphere pathway for the synthesis of tetrahydroquinolines (THQ) and N-methyltetrahydroquinoline (N-MTHQ).
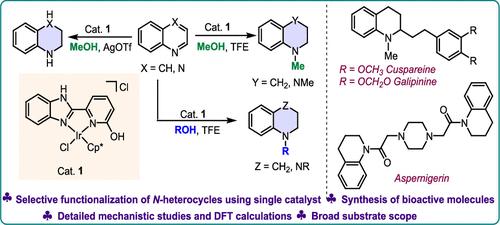
利用甲醇进行n -杂环的选择性还原和还原性甲基化
饱和n -杂环和n -甲基化或烷基化衍生物由于其多样化的生物和药理活性,在不同的科学领域至关重要。因此,实现它们的选择性地层是非常必要的,但需要创新的方法。本文报道了用甲醇作为双H2和甲基化源,Ir(III)催化n -杂环的选择性还原和n -甲基化。通过对反应参数的战略性调节,精确地控制了选择性,其中AgOTf对还原至关重要,2,2,2-三氟乙醇(TFE)促进了还原甲基化。该方法也被成功地推广到其他醇的还原性烷基化反应以及几种具有不同生物重要性的分子的合成。对照实验、动力学研究和密度泛函理论(DFT)计算进一步揭示了Ir(III)催化合成四氢喹啉(THQ)和n -甲基四氢喹啉(N-MTHQ)的外球途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


