Unveiling the Activation Pathway of the CO2 Reduction Catalyst trans(Cl)-[Ru(X,X′-dimethyl-2,2′-bipyridine)(CO)2Cl2] by Direct Spectroscopic Observation
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
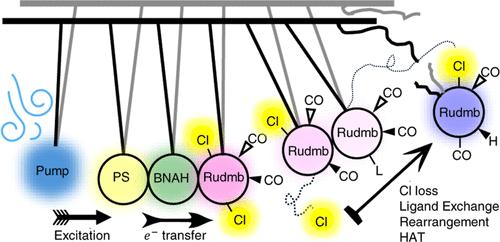
We report on the activation pathway of a series of CO2 reduction catalysts, trans(Cl)-[Ru(X,X′-dimethyl-2,2′-bipyridine)(CO)2Cl2], with a focus on trans(Cl)-[Ru(6,6′-dimethyl-2,2′-bipyridine)(CO)2Cl2]), in the presence of the reductive quencher 1-benzyl-1,4-dihydronicotinamide and the photosensitizer Ru(bpy)3Cl2. Most mechanistic studies of these types of catalytic systems use spectroelectrochemistry in the IR, where the vibrational frequencies of the carbonyl vibrations report on the electron density on the metal center. However, spectroelectrochemistry may miss short-lived intermediates, while at the same time the spectra can be dominated by accumulating side-products, which may play only a minor role in the reaction cycle. Transient IR spectroscopy on all relevant time scales, from picoseconds to hundreds of milliseconds, can bridge this gap, revealing a surprisingly complex reaction pathway (in combination with NMR spectroscopy as well as DFT calculations). That is, electron transfer from the reduced photosensitizer is followed by a loss of a first chloride ligand, a replacement of the second chloride ligand by a solvent molecule, and a ligand rearrangement that releases the strain between the equatorial carbonyl ligands and the methyl group on the bpy ligand in this catalyst. These reaction steps happen on a tens of nanoseconds to tens of microseconds time scale. In the case of trans(Cl)-[Ru(6,6′-dimethyl-2,2′-bipyridine)(CO)2Cl2]), the complex is then reduced a second time from the oxidized 1-benzyl-1,4-dihydronicotinamide on a significantly slower 10–100 ms time scale, protonated and the solvent ligand is exchanged back to a chloride. The final product hence is a hydride, RuII(6,6′-dmbpy)(CO)2ClH, which is stable on a minute-to-hour time scale. In case of trans(Cl)-[Ru(5,5′-dmbpy)(CO)2Cl2]), dimerization of the reduced species is possible, which eventually leads to the formation of cis(Cl)-[Ru(5,5′-dmbpy)(CO)2Cl2]. The work illustrates the power of transient IR spectroscopy to elucidate complex reaction pathways of such catalytic systems, and provides solid cornerstones for their kinetic control.
直接光谱观察揭示CO2还原催化剂反式(Cl)-[Ru(X,X ' -二甲基-2,2 ' -联吡啶)(CO)2Cl2]的活化途径
本文报道了一系列CO2还原催化剂反式(Cl)-[Ru(X,X′-二甲基-2,2′-联吡啶)(CO)2Cl2]在还原猝灭剂1-苄基-1,4-二氢烟酰胺和光敏剂Ru(bpy)3Cl2存在下的活化途径,重点研究了反式(Cl)-[Ru(6,6′-二甲基-2,2′-联吡啶)(CO)2Cl2]。这些类型的催化系统的大多数机理研究使用红外光谱电化学,其中羰基振动的振动频率报告了金属中心的电子密度。然而,光谱电化学可能会错过短寿命的中间体,而同时光谱可能被积累的副产物所主导,这些副产物在反应周期中可能只起很小的作用。瞬态红外光谱在所有相关的时间尺度上,从皮秒到数百毫秒,可以弥补这一差距,揭示出令人惊讶的复杂反应途径(结合核磁共振光谱和DFT计算)。也就是说,从被还原的光敏剂中转移电子之后,第一个氯配体丢失,第二个氯配体被溶剂分子取代,配体重排释放了该催化剂中赤道羰基配体和bpy配体上甲基之间的张力。这些反应步骤发生在几十纳秒到几十微秒的时间尺度上。在反式(Cl)-[Ru(6,6 ' -二甲基-2,2 ' -联吡啶)(CO)2Cl2]的情况下,配合物在10-100 ms的时间尺度上从氧化的1-苄基-1,4-二氢烟酰胺中第二次还原,质子化,溶剂配体交换回氯化物。因此,最终产物是一种氢化物,RuII(6,6 ' -dmbpy)(CO)2ClH,它在分钟到小时的时间尺度上是稳定的。在反式(Cl)-[Ru(5,5 ' -dmbpy)(CO)2Cl2]的情况下,还原物可能发生二聚化,最终形成顺式(Cl)-[Ru(5,5 ' -dmbpy)(CO)2Cl2]。这项工作说明了瞬态红外光谱在阐明这类催化系统的复杂反应途径方面的力量,并为其动力学控制提供了坚实的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


