Semirationally Engineering an Efficient P450 Peroxygenase for Regio- and Enantioselective Hydroxylation of Steroids
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
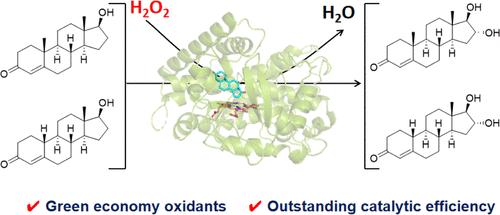
Enzymatic direct hydroxylation of unactivated C–H bonds in steroids provides a promising approach to enrich their structural and functional diversity, together with higher physiological and pharmacological activity. Here, we construct an efficient peroxide-driven P450 hydroxylase for the regio- and enantioselective hydroxylation of steroids. The NADH-dependent CYP154C5 monooxygenase is smoothly transformed into its peroxygenase mode by combining the strategies of H2O2 tunnel engineering and the introduction of a catalytic aspartate residue, which avoids the use of expensive nicotinamide cofactors and redox partner proteins. The variant F92A/R114A/E282A/T248D (AAA/T248D) quantitatively converted testosterone and nandrolone into the corresponding 16α-hydroxylation products, showing the best catalytic efficiency (kcat/Km) for testosterone hydroxylation among all known natural and engineered P450 peroxygenases to date. Crystal structural analysis and molecular dynamics simulations suggest that H2O2 tunnel engineering plays a crucial role in promoting the flow of H2O2 into active centers, and the introduced aspartate residue may participate in the activation of H2O2. Moreover, the milligram-scale preparation of 16α-hydroxytestosterone by AAA/T248D gave a substrate conversion rate (>98%) and an isolated yield (90%), suggesting potential for synthetic application. This work not only establishes a feasible semirational approach to engineered non-natural P450 peroxygenases but also provides a potentially practical approach for the enzymatic synthesis of hydroxylated steroid compounds.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


