Manganese Ions Chelated Tumosomes as Autologous Cancer Nanovaccines for Effective Suppression of Postsurgical Tumor Relapse
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
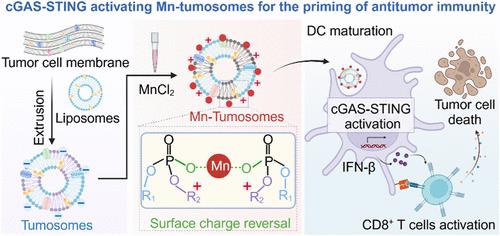
Autologous cancer vaccines represent a promising strategy to effectively suppress postoperative tumor relapse by eliciting tumor-specific immune responses that highly rely on the efficient internalization and lymph node-targeting delivery of vaccines. Herein, we report an autologous nanovaccine obtained by sequentially incorporating tumor plasma membrane proteins into liposomes, termed tumosomes, and chelating it with metallo-agonist of manganese ions. The yielded Mn-tumosomes with a positively charged surface exhibited significantly enhanced internalization by dendritic cells and enhanced lymph node targeting capacity, the latter of which is indicated by the near-infrared II fluorescence of silver sulfide nanoprobes labeled on their lipid bilayers. As a result, vaccination with Mn-tumosomes elicited potent tumor-specific CD8+ T cells to suppress the growth of challenged allogeneic tumors more effectively than vaccination via bolus injection of plain tumosomes and commercial immune agonists. Furthermore, with the excised tumor mass as the source of whole tumor cell antigens, the as-prepared autologous Mn-tumosomes effectively suppressed the growth of both residual tumor masses and spontaneously formed metastatic tumors, particularly in combination with anti-PD-1 immunotherapy. This work highlights a metal coordination based strategy to fabricate personalized whole-tumor cell nanovaccines with superior lymph node targeting and cellular uptake efficacy for the immunotherapeutic suppression of postoperative tumor relapse.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
文献相关原料
公司名称
产品信息
索莱宝
phosphate-buffered saline
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


