LncRNA CRCMSL interferes in phospholipid unsaturation to suppress colorectal cancer progression via reducing membrane fluidity
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
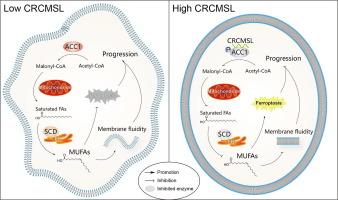
Reprogrammed metabolism is an important basis of colorectal cancer (CRC) progression; however, its mechanisms remain unclear. This study illustrated a novel mechanism for long noncoding RNA (lncRNA) CRCMSL in CRC, which was identified as a CRC suppressor in our previous study.Objective
To investigate whether CRCMSL suppresses colorectal cancer by interfering in lipid metabolism.Methods
Potential functions of CRCMSL were predicted by GSEA, which led to lipidomics. Ferroptosis process in CRC were evaluated by protein markers, probe-reported lipid peroxidation signals and transmission electron microscopy. Order and fluidity of phospholipid bilayers were detected by Laurdan generalized polarization (GP) assays and fluorescence recovery after photobleaching (FRAP) assays, respectively. RNA pull-down and RIP assays were performed to explore the target of CRCMSL. qPCR, western blot and enzyme activity detections were used to explore the effects of CRCMSL on the target. Orthotopic and subcutaneous xenografts in nude mice were used to validate efficacy of CRC in vivo.Results
CRCMSL-knockdown upregulated lipid synthesis and remodeled fatty acyl chains in phospholipids, inspiring studies on ferroptosis and phospholipid bilayers. CRCMSL-mediated biological processes and behaviors were restored by stearoyl-CoA desaturase (SCD), a key enzyme for the synthesis of monounsaturated fatty acids (MUFAs), suggesting that CRCMSL promotes ferroptosis and reduces membrane fluidity by interfering in phospholipid unsaturation. The target of CRCMSL in fatty acid metabolism is acetyl-CoA carboxylase 1 (ACC1), a key enzyme for de novo fatty acid synthesis. CRCMSL promoted ACC1 phosphorylation to restrict its activity. Firsocostat, an ACC oral inhibitor ND630, is a potential drug for CRC treatment in combination with CRCMSL.Conclusion
Our study illustrated a novel mechanism of CRCMSL-ACC1 axis-associated fatty acid metabolism in CRC progression, providing laboratory evidence for the development of targeted therapies for patients with advanced CRC.
导言程序化代谢是结直肠癌(CRC)进展的重要基础;然而,其机制仍不清楚。本研究揭示了长非编码 RNA(lncRNA)CRCMSL 在 CRC 中的新机制,在我们之前的研究中,CRCMSL 被鉴定为 CRC 抑制因子。方法通过 GSEA 预测 CRCMSL 的潜在功能,进而进行脂质组学研究。通过蛋白质标记物、探针报告的脂质过氧化信号和透射电子显微镜评估了 CRC 中的铁变态过程。磷脂双分子层的有序性和流动性分别通过劳尔丹广义极化(GP)测定和光漂白后荧光恢复(FRAP)测定进行检测。利用 qPCR、Western 印迹和酶活性检测来探讨 CRCMSL 对靶标的影响。结果 CRCMSL-敲除可上调脂质合成并重塑磷脂中的脂肪酰基链,从而激发了对铁变态反应和磷脂双层膜的研究。硬脂酰-CoA 去饱和酶(SCD)是合成单不饱和脂肪酸(MUFAs)的关键酶,它能恢复 CRCMSL 介导的生物过程和行为,这表明 CRCMSL 通过干扰磷脂的不饱和性促进了铁变态反应并降低了膜的流动性。CRCMSL 在脂肪酸代谢中的靶标是乙酰-CoA 羧化酶 1(ACC1),它是脂肪酸从头合成的关键酶。CRCMSL 可促进 ACC1 磷酸化,从而限制其活性。结论:我们的研究阐明了 CRCMSL-ACC1 轴相关脂肪酸代谢在 CRC 进展中的新机制,为开发针对晚期 CRC 患者的靶向疗法提供了实验室证据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


