Visible Light Boosted the Reduction of Cr(VI) in the Absence of Conventional Reductants in Frozen Solutions: The Overlooked Role of Inorganic Anions
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
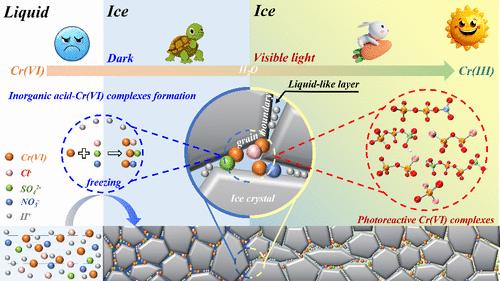
The conversion of Cr(VI) in frozen solutions is a mysterious process in the water environment. It has traditionally been believed that the reduction of Cr(VI) can only occur through interaction with traditional reducing agents like natural organic matters (NOMs) and Fe(II), with H2O merely acting as a solvent. However, this study reveals that visible light can induce the reduction of Cr(VI) by H2O even without conventional reducing agents. Specifically, within the narrow liquid-like layer between ice crystals, there is a significant increase in concentrations of protons, Cr(VI), and anions (Cl–, SO42–, and NO3–), which promotes the formation of various complexes between Cr(VI) and anions. DFT results reveal 11 types of stable Cr(VI)-anion complexes in solution, with six exhibiting visible light absorption properties. Quadrupole time-of-flight (QTOF) mass spectrometry confirms that the abundance of these six complexes (Cl–CrO2–Cl, Cr2O6–Cl, Cl–Cr2O5–Cl, Cr2O6–OSO3H, HO3SO–Cr2O5–OSO3H, and Cr2O6–ONO2) correlates with the extent to which visible light promotes Cr(VI) reduction, thus highlighting their crucial role as photoreactive intermediate complexes during this conversion process. These findings suggest that H2O in frozen solutions should no longer be regarded solely as a solvent but also as a reactant, thereby inspiring deeper insights into frozen solution chemistry.
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


