Myocardia-Injected Synergistically Anti-Apoptotic and Anti-Inflammatory Poly(amino acid) Hydrogel Relieves Ischemia-Reperfusion Injury
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
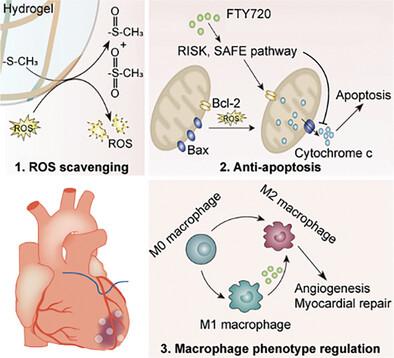
Reperfusion therapy is the most effective treatment for acute myocardial infarction, but its efficacy is frequently limited by ischemia-reperfusion injury (IRI). While antioxidant and anti-inflammatory therapies have shown significant potential in alleviating IRI, these strategies have not yielded satisfactory clinical outcomes. For that, a thermo-sensitive myocardial-injectable poly(amino acid) hydrogel of methoxy poly(ethylene glycol)45-poly(L-methionine20-co-L-alanine10) (mPEG45-P(Met20-co-Ala10), PMA) loaded with FTY720 (PMA/FTY720) is developed to address IRI through synergistic anti-apoptotic and anti-inflammatory effects. Upon injection into the ischemic myocardium, the PMA aqueous solution undergoes a sol-to-gel phase transition and gradually degrades in response to reactive oxygen species (ROS), releasing FTY720 on demand. PMA acts synergistically with FTY720 to inhibit cardiomyocyte apoptosis and modulate pro-inflammatory M1 macrophage polarization toward anti-inflammatory M2 macrophages by clearing ROS, thereby mitigating the inflammatory response and promoting vascular regeneration. In a rat IRI model, PMA/FTY720 reduces the apoptotic cell ratio by 81.8%, increases vascular density by 34.0%, and enhances left ventricular ejection fraction (LVEF) by 12.8%. In a rabbit IRI model, the gel-based sustained release of FTY720 enhanced LVEF by an additional 7.2% compared to individual treatment. In summary, the engineered PMA hydrogel effectively alleviates IRI through synergistic anti-apoptosis and anti-inflammation actions, offering valuable clinical potential for treating myocardial IRI.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


