Reactive Oxygen Species Inducing Triazolylpyridine-Based Ru(II)/Ir(III) Complexes for Therapeutically Enhanced Triple-Negative Breast Cancer Treatment
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
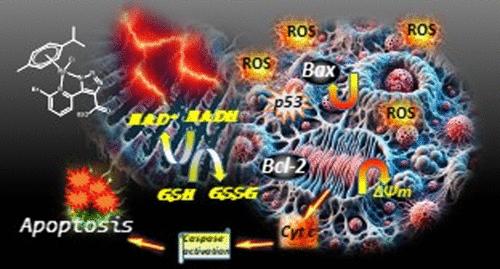
The effectiveness of existing systemic and targeted therapies remains limited in triple-negative breast cancer (TNBC) treatment. Much research has been conducted on reactive oxygen species (ROS)-mediated cancer cell death to overcome the shortcomings of the currently applied chemotherapeutic treatments. Herein, we have developed novel Ru(II)/Ir(III)-mediated triazolylpyridine complexes as ROS inducers. Upon entering the TNBC cells, the Ru(II) complex effectively accumulated in mitochondria and triggered the creation of ROS, facilitating dysfunction of mitochondria and oxidative DNA damage, ultimately causing death of cells through G2/M phase cell cycle arrest. Eventually, this complex induced the upregulation of BAX (pro-apoptotic protein) and downregulation of BCL-2 (antiapoptotic protein) and triggered the caspase 3/9 pathway and released cytochrome c in the cytosol for apoptosis. The complex JRu (RuII triazolylpyridine) significantly reduced the integrity and viability of TNBC 3D spheroids.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


