Liquid-like condensates that bind actin promote assembly and bundling of actin filaments
IF 10.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
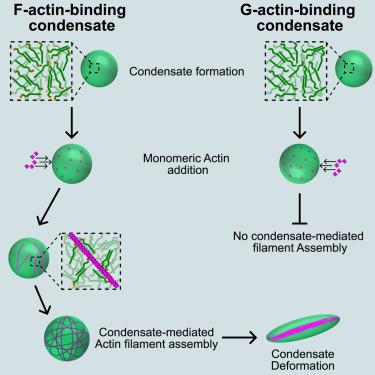
Biomolecular condensates perform diverse physiological functions. Previous work showed that VASP, a processive actin polymerase, forms condensates that assemble and bundle actin. Here, we show that this behavior does not require proteins with specific polymerase activity. Specifically, condensates composed of Lamellipodin, a protein that binds actin but is not an actin polymerase, were also capable of assembling actin filaments. To probe the minimum requirements for condensate-mediated actin bundling, we developed an agent-based computational model. Guided by its predictions, we hypothesized that any condensate-forming protein that binds filamentous actin could bundle filaments through multivalent crosslinking. To test this, we added a filamentous-actin-binding motif to Eps15, a condensate-forming protein that does not normally bind actin. The resulting chimera formed condensates that facilitated efficient assembly and bundling of actin filaments. Collectively, these findings broaden the family of proteins that could organize cytoskeletal filaments to include any filamentous-actin-binding protein that participates in protein condensation.
求助全文
约1分钟内获得全文
求助全文
来源期刊
Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


