Versatile and Controlled Synthesis of Degradable, Water-Soluble Bottlebrush Polymers with Poly(disulfide) Backbones Derived from α-Lipoic Acid
IF 5.2
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
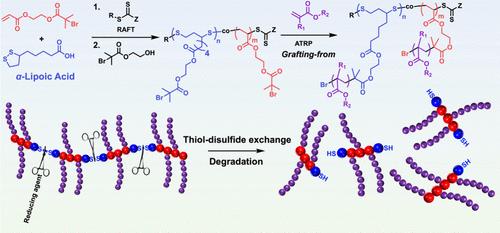
Bottlebrush (BB) polymers, with their densely grafted side chains and unique architecture, are highly advantageous for drug delivery due to their high functional group density for drug conjugation, unimolecular nature, and enhanced biodistribution properties. These attributes enable extended blood circulation half-life, improved tumor tissue penetration, and high tumoral drug accumulation. However, the typically nondegradable, all-carbon backbones of most BB polymers limit their suitability for applications requiring controlled clearance and biodegradability. To address this, we developed degradable BB polymers with poly(disulfide) backbones synthesized via reversible addition–fragmentation chain transfer (RAFT) copolymerization of α-lipoic acid (LA), a renewable and readily available compound, with acrylate-based inimers. These copolymers feature degradable backbones and initiating sites for subsequent BB synthesis. Using an atom transfer radical polymerization (ATRP) grafting-from methodology, we synthesized BB polymers with relatively low dispersities (Đ = 1.30–1.53), high backbone degrees of polymerization (DPbb), and high molar masses (Mn,MALS = 650–2700 kg/mol). The easily cleavable disulfide bonds enabled backbone degradation under mild reducing conditions. Beyond hydrophilic BB with tri(ethylene glycol) methyl ether acrylate (TEGA) side chains, we synthesized BB with cationic, anionic, and zwitterionic side chains, demonstrating broad monomer compatibility. This scalable approach produces water-soluble, degradable BB polymers with tunable architectures and predictable molecular weights. By addressing the need for degradability in BB polymers, this work advances their potential for drug delivery, offering enhanced functionality, biocompatibility, and sustainability.
α-硫辛酸基可降解水溶性瓶刷聚合物的通用可控合成
瓶刷(BB)聚合物具有密集接枝的侧链和独特的结构,由于其高官能团密度用于药物偶联,单分子性质和增强的生物分布特性,对药物递送非常有利。这些特性可以延长血液循环半衰期,改善肿瘤组织渗透,提高肿瘤药物积累。然而,大多数BB聚合物的典型不可降解的全碳骨架限制了它们在需要控制清除和生物降解性的应用中的适用性。为了解决这个问题,我们开发了具有聚(二硫)骨架的可降解BB聚合物,通过α-硫辛酸(LA)的可逆加成-破碎链转移(RAFT)共聚合成。α-硫辛酸是一种可再生且易于获得的化合物。这些共聚物具有可降解的骨架和引发位点,可用于随后的BB合成。采用原子转移自由基聚合(ATRP)接枝的方法,我们合成了相对低分散度(Đ = 1.30-1.53)、高主链聚合度(DPbb)和高摩尔质量(Mn,MALS = 650-2700 kg/mol)的BB聚合物。易于切割的二硫键使主链在温和的还原条件下降解。除了具有三(乙二醇)甲基醚丙烯酸酯(TEGA)侧链的亲水性BB外,我们还合成了具有阳离子、阴离子和两性离子侧链的BB,证明了广泛的单体相容性。这种可扩展的方法生产水溶性、可降解的BB聚合物,具有可调的结构和可预测的分子量。通过解决BB聚合物可降解性的需求,这项工作提高了它们在药物输送方面的潜力,提供了增强的功能、生物相容性和可持续性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.40
自引率
3.40%
发文量
209
审稿时长
1 months
期刊介绍:
ACS Macro Letters publishes research in all areas of contemporary soft matter science in which macromolecules play a key role, including nanotechnology, self-assembly, supramolecular chemistry, biomaterials, energy generation and storage, and renewable/sustainable materials. Submissions to ACS Macro Letters should justify clearly the rapid disclosure of the key elements of the study. The scope of the journal includes high-impact research of broad interest in all areas of polymer science and engineering, including cross-disciplinary research that interfaces with polymer science.
With the launch of ACS Macro Letters, all Communications that were formerly published in Macromolecules and Biomacromolecules will be published as Letters in ACS Macro Letters.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


