Targeting gut S. aureofaciens Tü117 serves as a new potential therapeutic intervention for the prevention and treatment of hypertension
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Currently, the regulation of specific gut microbial metabolism for the development and/or treatment of hypertension remains largely unexplored. Here, we show that α-lipomycin, produced by Streptomyces aureofaciens (S. aureofaciens) Tü117, is upregulated in the serum of high-salt diet (HSD) mice and patients with essential hypertension. α-lipomycin causes vasodilation impairment involving transient receptor potential vanilloid 4 (TRPV4)-mediated nitric oxide and endothelium-derived hyperpolarizing factor pathways in mice. We also find that Lactobacillus plantarum (L. plantarum) CCFM639 attenuates the increase in blood pressure (BP) potentially through inhibiting the proliferation of S. aureofaciens Tü117 in mice. An exploratory intervention trial indicates that L. plantarum CCFM639 supplementation reduces BPs in subjects newly diagnosed with pre-hypertension or stage 1 hypertension without antihypertensive medication. Our findings provide evidence for a role of S. aureofaciens Tü117-associated α-lipomycin elevation in the pathogenesis of HSD-induced hypertension, highlighting that targeting gut bacteria serves as a new therapeutic intervention for hypertension.
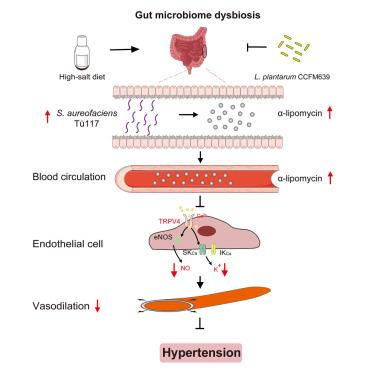
靶向肠道金黄色葡萄球菌 117为预防和治疗高血压提供了一种新的治疗干预手段
目前,特定肠道微生物代谢对高血压的发展和/或治疗的调节在很大程度上仍未被探索。本研究表明,高盐饮食(HSD)小鼠和原发性高血压患者血清中由金黄色链霉菌(S. aureofaciens) 117产生的α-lipomycin上调。α-脂霉素引起小鼠血管舒张损伤,涉及瞬时受体电位香草素4 (TRPV4)介导的一氧化氮和内皮源性超极化因子途径。我们还发现植物乳杆菌(L. plantarum) CCFM639可能通过抑制金黄色葡萄球菌 117在小鼠体内的增殖来降低血压(BP)的升高。一项探索性干预试验表明,植物乳杆菌CCFM639补充剂可以降低新诊断为高血压前期或1期高血压而不服用降压药的受试者的血压。我们的研究结果为金黄色葡萄球菌 117相关α-脂霉素升高在hsd诱导的高血压发病机制中的作用提供了证据,强调靶向肠道细菌可作为一种新的高血压治疗干预手段。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


