Atomic Hydrogen in Hydrogenolysis: Converting and Detoxifying Carbon-Heteroatom Bonds via Paired Electrolysis
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
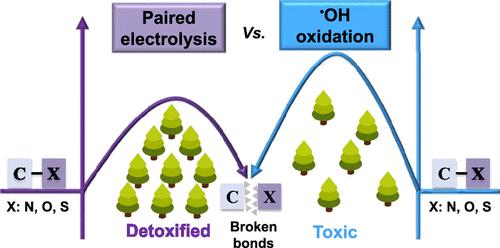
The presence of carbon-heteroatom bonds (C–N, C–O, and C–S) significantly enhances the stability and toxicity of pollutants. Hydroxyl radicals (•OH)-mediated electrochemical processes show promise; however, the bond energies associated with carbon-heteroatom bonds exceed 200 kJ/mol, which constrains the effectiveness of oxidative degradation and detoxification. We have developed a paired electrolysis process coupling hydrogen atom (H*) generation at the cathode with •OH production at the anode. The involvement of H* and •OH in this system was first confirmed by using methylene blue (MB) as an electrochemical probe. When applied to the degradation of glyphosate (GP), which contains C–N bonds, the paired electrolysis process achieved removal efficiencies for COD, TOC, and toxicity that were twice those of individual oxidation processes. The degradation kinetics also exhibited performance that was double that of individual oxidation processes. Mass spectrometry and theoretical calculations confirmed that hydrogenolysis of H* effectively attacks high-energy C–N bonds, thereby circumventing the rate-limiting steps associated with standalone •OH oxidation, enhancing pollutant degradation and reducing toxicity. When applied to pollutants containing C–O and C–S bonds, the paired electrolysis process demonstrated improvements in COD, TOC, and toxicity removal of approximately 30%, 10%, and 20%, respectively, showcasing its multifunctionality and scalability. Seven days of practical wastewater experiments further validated the effectiveness and durability of this technology.
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


