Sulfidation of Magnetite for Superior Dechlorination of Trichloroethene
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
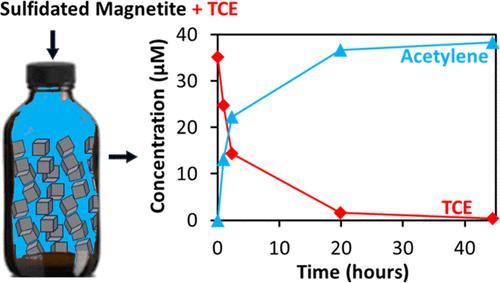
The reported contributions of magnetite to the abiotic natural attenuation of chlorinated ethenes have generated interest in its potential for soil and groundwater remediation. In this study, we investigated the impact of the two-step sulfidation method on the physicochemical properties and reactivity of magnetite with trichloroethene (TCE). We systematically evaluated the effect of different sulfur precursors (dithionite, thiosulfate, and sulfide) and sulfur-to-iron ([S/Fe]dosed) molar ratios on the reactivity. Results were compared to those of sulfidated nZVI (S-nZVI) as a benchmark for assessing the efficacy of sulfidated magnetite (S–Fe3O4). The findings indicated limited reactivity of magnetite when sulfidated with dithionite and thiosulfate. However, sulfidation with sulfide yielded reaction rates comparable to those of S-nZVI, particularly at lower [S/Fe]dosed ratios. At higher [S/Fe]dosed ratios (>0.1), sulfide-sulfidated magnetite (S–Fe3O4_S) exhibited reaction rates surpassing those of S-nZVI, with the major dechlorination product being acetylene. Nonetheless, reusability experiments demonstrated that the performance of S–Fe3O4 diminished with aging. These results show that S–Fe3O4_S achieved complete transformation of TCE to acetylene, with reaction rates comparable to S-nZVI. Given its lower cost of production, engineered S–Fe3O4_S remediation systems could serve as a more affordable alternative for in situ chemical reduction of TCE with further research and development.
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


