Shear Viscosity of Short-Chain Imidazolium Ionic Liquids from Equilibrium and Nonequilibrium Molecular Dynamics: Atomistic and Coarse-Grained Levels
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
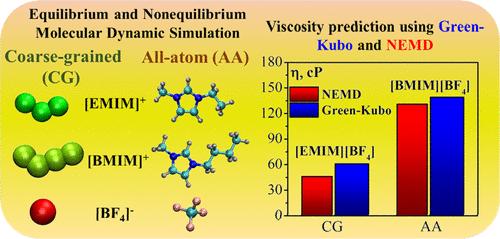
The ability to predict transport properties, such as viscosity, through molecular simulation is a valuable advantage. Obtaining reliable estimates for these properties can be more challenging compared to equilibrium properties. Our manuscript focuses on an in-depth discussion of two prominent methods for predicting transport properties: equilibrium molecular dynamics (EMD) using the Green–Kubo approach and nonequilibrium molecular dynamics (NEMD) with the periodic perturbation method. These methods were utilized to calculate the shear viscosity of highly viscous room-temperature ionic liquids (RTILs), specifically 1-ethyl-3-methylimidazolium tetrafluoroborate [EMIM][BF4] and 1-butyl-3-methylimidazolium tetrafluoroborate [BMIM][BF4]. Additionally, we assessed both all-atom and coarse-grained models for describing the RTILs, as well as examined the impact of various parameters such as barostat, ensemble, acceleration step, length, and number of trajectories on viscosity calculation. Our analysis revealed that the NEMD method provided the most accurate predictions of shear viscosity for short-chain imidazolium ionic liquids. The influence of barostat and ensemble choice was found to be minimal, and reducing the acceleration step in the NEMD method can help decrease simulation time while maintaining accuracy.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


