Chemo-Selective Electrochemical Pinacol Coupling of Aldehydes and Ketones Using TMSN3 as a Promoter
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The pinacol coupling is a significant method for carbon–carbon bond formation. Here, we present an efficient electrochemical approach for pinacol coupling of aldehydes and ketones using TMSN3 as a sacrificial reagent. This method exhibits broad applicability to aryl, heteroaryl, and alkyl aldehydes/ketones with excellent chemo-selectivity and high yields (40 examples, up to 99% yield) under mild conditions. This method can also be applied in the selective reduction of phthalimides to hydroxyl lactams in good yields. The proposed mechanism was elucidated by control experiments and cyclic voltammetry.
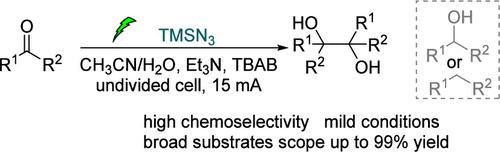
以TMSN3为启动子的醛酮化学选择性电化学偶联
醇偶联是碳-碳键形成的重要方法。在这里,我们提出了一种有效的电化学方法,以TMSN3作为牺牲试剂进行醛和酮的蒎醇偶联。该方法广泛适用于芳基、杂芳基和烷基醛/酮,在温和条件下具有优异的化学选择性和高收率(40个样品,收率高达99%)。该方法也可用于邻苯二甲酸亚胺选择性还原成羟基内酰胺,收率较高。通过对照实验和循环伏安法对其机理进行了验证。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


