Revealing Mechanisms of an Eco-Friendly GaN Electrochemical Mechanical Removal Process Modified with Green Fenton Reaction
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
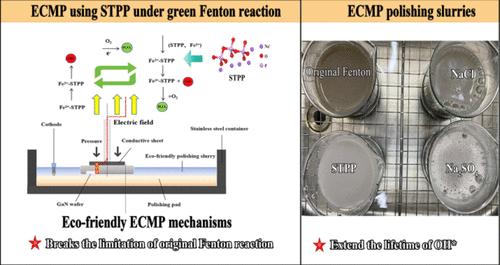
Gallium Nitride (GaN), a leading third-generation semiconductor material, offers exceptional properties and broad application potential. However, its high hardness and chemical inertness make GaN wafers difficult to process, posing significant challenges in improving the polishing efficiency. During the electrochemical mechanical polishing (ECMP) process, the oxidation effect on the GaN surface critically affects the material removal rate (MRR). While various methods have been developed to enhance the concentration of hydroxyl radicals (OH*) to improve the process, their instability and rapid decomposition present further obstacles to maintaining concentration and stability. The present study proposed a novel approach using sodium tripolyphosphate (STPP) as an electrolyte additive for ECMP under a green Fenton reaction. Furthermore, the MRR and surface roughness (Ra) of GaN-ECMP were comprehensively evaluated. Atomic force microscopy (AFM) was used to observe the surface morphologies of GaN wafers after ECMP. Energy-dispersive spectrometry (EDS) and X-ray Photoelectron Spectroscopy (XPS) were used to characterize the generated oxide layers. The nanoscratch tests were conducted to reveal the material removal mechanisms of GaN under the synergistic effect of STPP and Fe2+. The results indicated that an efficient GaN-ECMP process (MRR > 800 nm/h) with good surface quality (Ra < 0.33 nm) can be realized by employing STPP as electrolyte additives. This study breaks the limitation of iron precipitate formation in the ECMP slurries during the Fenton reaction. It extends the lifetime of the hydroxyl radicals (OH*) produced by the Fenton reaction, which presents a beneficial exploration of seeking an eco-friendly polishing slurry for GaN-ECMP.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


