Intraparticle Diffusion Behavior of Rhodamine 6G in Single Silica Particle Revealed by Fluorescence Correlation Spectroscopy
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
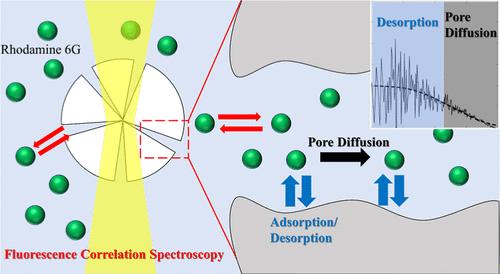
Understanding the intraparticle diffusion mechanism within single particles is crucial for processes such as chromatographic separation, drug delivery, and solid extraction. However, when the time required to reach distribution equilibrium is extremely short (on the order of several seconds), conventional kinetic detection methods pose significant challenges in observing intraparticle diffusion behavior. In this study, we employed fluorescence correlation spectroscopy (FCS)─a technique capable of detecting diffusion behavior at equilibrium─to investigate the intraparticle diffusion of rhodamine 6G (Rh6G) within single porous silica particles of varying pore sizes. The autocorrelation coefficients of Rh6G were fitted using two-component analysis, revealing faster and slower diffusion components associated with the pore diffusion, without and with adsorption/desorption, respectively―behaviors that were not observed by the kinetic method described by our previous study. Further analysis of the slower diffusion component was conducted using pore and surface diffusion models. Our findings indicate that pore diffusion is the primary diffusion mechanism for Rh6G within silica particles. Thus, we demonstrated that the intraparticle diffusion mechanism of Rh6G in silica particles can be elucidated using FCS measurements.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


