Integrating hydroformylations with methanol-to-syngas reforming
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Commodity chemical production is heavily dependent on fossil feedstocks. Transitioning to renewable resources is a pressing necessity, with green methanol being a promising candidate for rethinking chemical platforms. Here, we report how interlocking methanol-to-syngas reforming and hydroformylation of olefins may integrate methanol as a platform for accessing renewable oxo-products. This study demonstrates the importance of interlocking kinetics and selectivity of a ruthenium-catalyzed acceptorless dehydrogenation and a rhodium-catalyzed hydroformylation. Notably, coal- or natural gas-derived syngas can be substituted with fuel-grade e-methanol obtained from captured CO2 and green hydrogen. Although these conditions do not replicate large-scale industrial settings, we consider this dual-catalysis approach a proof of concept illustrating the potential to synthesize oxo-products entirely from CO2-derived methanol. We envision that redesigning chemical value chains to extend from renewable platforms like methanol could play a pivotal role toward establishing a more sustainable chemical industry.
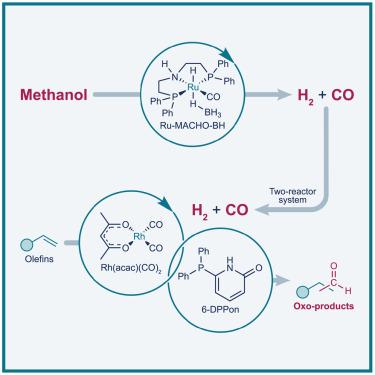

氢甲酰化与甲醇合成气重整相结合
商品化工生产严重依赖化石原料。向可再生资源过渡是迫切需要的,绿色甲醇是重新思考化学平台的有希望的候选者。在这里,我们报告了联锁甲醇制合成气重整和烯烃的氢甲酰化如何将甲醇作为获取可再生氧产物的平台。本研究证明了钌催化的无受体脱氢和铑催化的氢甲酰化联锁动力学和选择性的重要性。值得注意的是,煤或天然气衍生的合成气可以用从捕获的二氧化碳和绿色氢中获得的燃料级e-甲醇取代。虽然这些条件不能复制大规模的工业环境,但我们认为这种双催化方法证明了完全从二氧化碳衍生的甲醇合成氧产物的潜力。我们设想,重新设计化学品价值链,从甲醇等可再生平台延伸,可以在建立一个更可持续的化学工业方面发挥关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


