Systematic evolution of functional oligonucleotides for targeted protein degradation
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
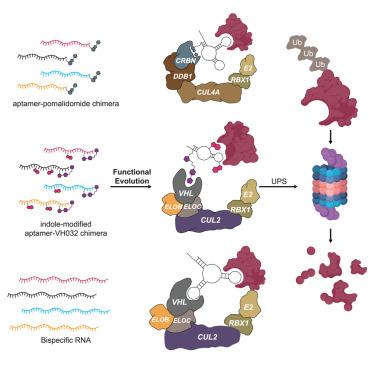
Targeted protein degradation (TPD) technologies leveraging the ubiquitin-proteasome system address “undruggable” proteins but struggle with optimizing targeting warheads and linkers. This study introduces a systematic platform for creating aptamer-based TPD molecules on demand. We developed a microbead-displayed oligonucleotide-E3 ligand chimera library and applied in vitro ubiquitination systems by using a fluorescent assay with bead sorting to identify high-affinity aptamer-chimera degraders that bind to target proteins and recruit E3 ligase for ubiquitination. This approach, tested with CRBN and VHL E3 ligases, successfully degraded BRD4 and IRAK4 proteins. Additionally, we evolved a bispecific RNA aptamer degrader, demonstrating the versatility of our platform. The selected aptamer chimeras achieved degradation rates of up to 87% for BRD4. Functional assays showed effective inhibition of cancer cell proliferation, induction of apoptosis, and significant tumor growth suppression in a subcutaneous tumor model. These findings highlight the potential of aptamer-based TPD technologies as powerful tools for cancer treatment.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


